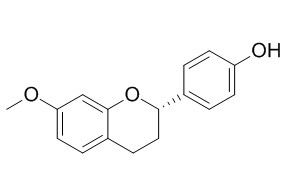
4'-Hydroxy-7-methoxyflavan
4'-Hydroxy-7-methoxyflavan shows an important cytotoxic effect against human leukemic Molt 4 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Preprints2022, 202211.0388.v1.
Curr Issues Mol Biol.2024, 46(4):3328-3341.
J Microbiol Biotechnol.2024, 35:e2408022.
Genes Environ.2024, 46(1):13.
Mol Divers.2022, s11030-022-10586-3.
J of the Korean Society of Cosmetics and Cosmetology2019, 225-231
Plant Direct.2021, 5(4):e00318.
Phytomedicine2022, 104:154318
Int J Mol Sci.2022, 23(20):12516.
Sci Rep. 2018, 1-9
Related and Featured Products
Planta Med. 1991 Oct;57(5):437-9.
Cytotoxic activity of Amaryllidaceae alkaloids from Crinum augustum and Crinum bulbispermum.[Pubmed:
1798796 ]
METHODS AND RESULTS:
The cytotoxic activity of five minor Amaryllidaceae alkaloids and one flavan isolated from Crinum augustum Rox and Crinum bulbispermum Milne were tested on human leukemic Molt 4 cells.
CONCLUSIONS:
Whereas the crinine-type alkaloids (6 alpha-hydroxycrinine, powelline) and the new type augustamine did not even inhibit the growth of Molt 4 cells, the lycorine-type alkaloid (pratorinine) and the crinine-type alkaloid (6 alpha-hydroxybuphanisine) showed a moderate cytotoxic activity and the flavan (4'-Hydroxy-7-methoxyflavan) showed an important cytotoxic effect.
Nat Prod Res. 2016;30(7):761-7.
A new flavan from the Drynaria bonii H. Christ rhizomes.[Pubmed:
26230303 ]
In Vietnam, the medicinal plant Drynaria bonii H. Christ is used for the treatment of osteoporosis, bone fractures, and stimulate the growth of hair, treat tinnitus (Ho 2002; Loi 2004).
METHODS AND RESULTS:
In this article, experiments were designed to investigate the proliferation activity of ethanol, n-hexane, chloroform, ethyl acetate and methanol extracts from D. bonii rhizomes on MG-63 human osteoblast-like cells. The results showed that methanol and hexane extracts had the ability to proliferate MG-63 cells at the concentration varying from 0.1 to 0.01 μg/mL. In particular, at the concentration of 0.01 μg/mL, hexane and methanol extracts illustrated the highest proliferation ratio with 9.31% and 6.16%, respectively.
CONCLUSIONS:
By column chromatography, a new compound named drynaether A (1) and five known compounds uracil (2), 4'-Hydroxy-7-methoxyflavan (3), kaempferol (4), indole-3-carboxylic acid (5) and protocatechuic acid (6) were isolated and identified from the methanol extract.
J Nat Prod. 2001 Feb;64(2):214-6.
Studies on flavans. 1. Facile synthesis of (+/-)-7-hydroxy-3',4'-methylenedioxyflavan and (+/-)-4'-hydroxy-7-methoxyflavan by a BF3.Et2O-mediated pyran cyclization.[Pubmed:
11430003]
METHODS AND RESULTS:
A facile approach for the synthesis of flavans was developed by employing a BF3.Et2O-catalyzed pyran cyclization in an aprotic polar solvent as a key step, by which concise total syntheses of (+/-)-7-hydroxy-3',4'-methylenedioxyflavan (1) and (+/-)-4'-Hydroxy-7-methoxyflavan (2), two naturally occurring flavans, were achieved.