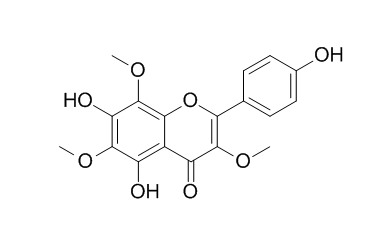
4',5,7-Trihydroxy 3,6,8-trimethoxyflavone showed significant cytotoxic activities in several mammalian cell lines, it also displayed interesting anti-HIV activity in the syncytium assay.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Tetrahedron, 2002, 58(40):8073-8086.
Cytotoxic and anti-HIV-1 constituents of Gardenia obtusifolia and their modified compounds.[Reference:
WebLink]
METHODS AND RESULTS:
5α-Cycloart-24-ene-3,23-dione (1), 5α-cycloart-24-ene-3,16,23-trione (2) and methyl 3,4-seco-cycloart-4(28),24-diene-29-hydroxy-23-oxo-3-oate (3), together with five known flavones 5,7,4′-trihydroxy-3,8-dimethoxyflavone (4), 5,7,4′-trihydroxy-3,8,3′-tri-methoxyflavone (5), 5,7,4′-trihydroxy-3,6,8-trimethoxyflavone (4',5,7-Trihydroxy 3,6,8-trimethoxyflavone,6), 5,4′-dihydroxy-3,6,7,8-tetramethoxyflavone (7) and 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (8) have been isolated from the leaves and twigs of Gardenia obtusifolia. The structures were assigned on the basis of spectroscopic methods.
CONCLUSIONS:
Compounds 3–8 and some of the modified compounds showed significant cytotoxic activities in several mammalian cell lines, especially 8 and its diacetate 21 which exhibited potent cytotoxicities (compound 8: P-388 0.05 μg/mL, KB 0.09 μg/mL, BCA-1 0.63 μg/mL, Lu-1 0.09 μg/mL, ASK 0.70 μg/mL; its diacetate: P-388 0.27 μg/mL, KB 0.06 μg/mL, BCA-1 0.53 μg/mL, Lu-1 0.49 μg/mL). It was also found that 5, 8 and 21 showed antimitotic acitivity in the ASK assay. Compounds 2, 4, 6, 7 and some of the modified compounds displayed interesting anti-HIV activity in the syncytium assay, but were inactive or exhibited weak activity in the HIV-1 RT assay; while compound 3 was found to be active in the HIV-1 RT assay (99.9 % inhibition at 200 μg/mL), but cytotoxic in the syncytium assay.