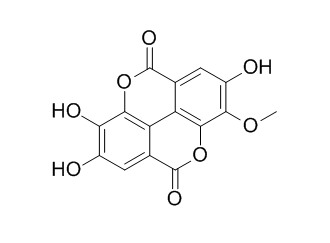
3-Methyl ellagic acid
3-Methyl ellagic acid has anti-oxidant activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nat Plants.2016, 3:16205
Anticancer Res.2018, 38(4):2127-2135
J Mol Histol.2019, 50(4):343-354
Int J Mol Sci.2020, 21(9):3239.
Toxicol In Vitro.2022, 81:105346.
Phytomedicine.2023, 120:155063.
Journal of Apicultural Research2021, 60(1)
Pharmacol Res.2022, 182:106346.
Int J Pharm.2022, 618:121636.
J Adv Res.2021, 35:245-257.
Related and Featured Products
Phytochemistry, 1996, 41(3):775-778.
Ellagic acid derivatives and naphthoquinones of Dionaea muscipula from in vitro cultures.[Reference:
WebLink]
METHODS AND RESULTS:
From Dionaea muscipula, obtained by in vitro culture, the known compounds plumbagin, chloroplumbagin and 8,8′-biplumbagin as naphthoquinones, 1-O-β-galloylglucose, ellagic acid,
3-O-methylellagic acid(3-Methyl ellagic acid), 3,3′-di-O-methylellagic acid and its 4-O-glucoside, and a new compound, the 4,4′-di-O-glucoside of 3,3′-di-O-methylellagic acid, were isolated.
CONCLUSIONS:
The assignments of NMR resonances of 3,3′-di-O-methylellagic acid 4-O-glucoside were substantiated by correlation techniques.
Journal of Medicinal Plant Research (2011) 5(23) 5584-5589.
Antioxidant and cytotoxic activity of the acetone extracts of root of Euphorbia hylonoma and its ellagic acid derivatives.[Reference:
WebLink]
METHODS AND RESULTS:
The acetone extracts and fractions prepared from the roots of Euphorbia hylonoma were investigated for 1, 1-diphenyl-2-pycrylhydrazyl (DPPH) free radical, superoxide anion and hydroxyl radical scavenging activity and cytotoxic activity against the human hepatoma cell line SMMC-7721, human cervix epitheloid carcinoma cell line HeLa and the human gastric cancer cell line SGC-7901. The acetone extract and fraction of EtOAc showed high antioxidant activities. The acetone extract and fraction of water showed a stronger cytotoxic effect on all tested cells, with IC50 values being less than 20 μg/mL. The acetone extract and fraction of water induced cell cycle arrest in G1 phase in SMMC-7721 cells.
CONCLUSIONS:
The major compounds isolated from the acetone extract were ellagic acid derivatives and showed weak cytotoxic activities on the tested cell lines, and these compound were 3,3',4-tri-O-methylellagic acid (1), 3,3'-di-O-methylellagic acid (2), 3-O-methylellagic acid (3-Methyl ellagic acid, 3) and 3,3'-di-O-methylellagic acid-4'-O-β-dxylopyranosid (4). Compound 3 showed better obvious radical scavenging activity than other tested compounds.