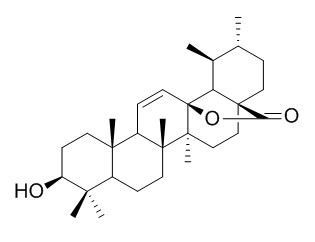
3-Hydroxy-11-ursen-28,13-olide
3β-Hydroxy-urs-11-en-28,13β-olide shows a potent concentration-independent cytoprotective effect against CCl4-induced injury on human hepatoma cell line relative to silymarin as a reference standard. It exhibits weak-moderate antiproliferative activity against the A2780 human ovarian cancer cell line.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Immunol. 2016, 78:121-132
Int J Mol Sci.2024, 25(18):9909.
Biochem Pharmacol.2017, 130:10-20
J.Korean Soci. Food Sci. Nutri.2024, 53(11):1166-1177
Mol Med Rep.2014, 9(5):1653-9
Pharmaceuticals (Basel).2024, 17(9):1130.
Korean J. Medicinal Crop Sci.2023, 31(6):388-395.
Nutrients.2023, 15(24):5020.
In Vitro Cellular & Developmental Biology - Plant2022, 58:972-988.
Naunyn Schmiedebergs Arch Pharmacol.2021, 394(1):107-115.
Related and Featured Products
Phytochem. Lett.,2011,4(4):421-5.
Ovarian antiproliferative activity directed isolation of triterpenoids from fruits of Eucalyptus camaldulensis Dehnh.[Reference:
WebLink]
METHODS AND RESULTS:
Bioassay-guided fractionation of a methanol extract of the fruits of Eucalyptus camaldulensis Dehnh. with moderate antiproliferative activity afforded the new triterpene, 3β-acetoxy-urs-11,13(18)-dien-28-oic acid (1) along with the known triterpenoids 3β-hydroxy-urs-11-en-28,13β-olide (3-Hydroxy-11-ursen-28,13-olide,2), 3β-acetoxy-urs-11-en-28,13β-olide (3-Acetoxy-11-ursen-28,13-olide ,3), 3-acetylbetulinic acid (4), oleanolic acid (5), ursolic acid (6), β-amyrin acetate (7), β-sitosterol (8) and sitosterol 3-O-β-D-glucopyranoside (9). Their structures were established on the basis of analysis of their 1H and 13C NMR, APT, gHSQC, gHMBC, UV, IR and mass spectral data.
CONCLUSIONS:
The tested triterpenoids (1–7) exhibited weak-moderate antiproliferative activity against the A2780 human ovarian cancer cell line.
Nat Prod Res. 2015;29(5):423-9.
A new triterpene and protective effect of Periploca somaliensis Browicz fruits against CCl₄-induced injury on human hepatoma cell line (Huh7).[Pubmed:
25179815 ]
METHODS AND RESULTS:
The potential hepatoprotective effect of the methanolic extract of Periploca somaliensis Browicz fruits, its different fractions (n-hexane, chloroform and n-butanol) and the major isolated compound ursolic acid was evaluated using the human hepatoma cell line (Huh7) based on the changes in the activity of aspartate aminotransferase, alanine transaminase, glutathione and superoxide dismutase. Each sample was tested at three different concentrations (1000, 100 and 10 μg/mL). All tested samples exhibited a potent concentration-independent cytoprotective effect relative to silymarin as a reference standard. Chromatographic fractionation of the chloroform-soluble fraction of the methanol extract of P. somaliensis Browicz fruits afforded two known triterpenes, namely ursolic acid, and 11α,12α-epoxy-3β-hydroxy-olean-13β,28-olide, and a newly discovered one, namely 3β-hydroxy-urs-11-en-13β,28-olide(3-Hydroxy-11-ursen-28,13-olide).
CONCLUSIONS:
The structures of the isolated compounds were elucidated by the analysis of 1D and 2D NMR spectral data.