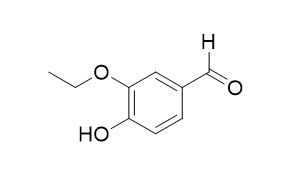
3-Ethoxy-4-hydroxybenzaldehyde
3-Ethoxy-4-hydroxybenzaldehyde is a new vir -inducer. It shows acetylcholinesterase inhibition.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Phytopathology2021, 169,Issue11-12.
Applied Biological Chemistry2021, 64(4)
Nat Plants.2016, 3:16205
Appl. Sci.2020, 10(20), 7323.
Int J Mol Sci.2022, 23(5):2796.
Cell Mol Biol(Noisy-le-grand)2019, 65(7):77-83
Turk J Med Sci.2023 53: 1312-1320.
iScience.2024, 27(8):110496.
Cell Prolif.2021, 54(8):e13083.
Toxins (Basel).2023, 15(3):231.
Related and Featured Products
Biological Mass Spectrometry, 2010, 12(4):163-169.
Identification of urinary 3‐ethoxy‐4‐hydroxybenzoic and 3‐ethoxy‐4‐hydroxymandelic acids after dietary intake of ethyl vanillin.[Reference:
WebLink]
The activation of the Agrobacterium virulence system is known to be induced by certain phenolic compounds.
METHODS AND RESULTS:
We have tested the vir-inducing ability of fifty compounds, by using a virB-lacZ gene fusion, and analysed the relationship between structure and activity of these compounds. In this way we have identified several new vir -inducers: coniferylalcohol, 3,5-dimethoxy-4-hydroxybenzene, homovanillic acid, ferulic acid, 3-Ethoxy-4-hydroxybenzaldehyde and guaiacol, all of which are compounds with strong or moderate activity and four compounds with weak vir -inducing activity. In view of the specificity of vir -inducers, our data extended observations of others and enabled us to define the specific structural features of a vir -inducer molecule. In addition we show here that induction of the octopine Ti vir -genes is (i) optimal at 29° C and totally abolished at 37° C., and (ii) strongly inhibited at low concentrations of sodium chloride. The implications for plant transformation are discussed.
Phytochemical Analysis Pca, 2003,14(3):127-31.
Specificity of signal molecules in the activation of Agrobacterium virulence gene expression.[Reference:
WebLink]
A method has been developed to determine the false-positive effects on acetylcholinesterase inhibition in the TLC assay based on Ellman's method.
METHODS AND RESULTS:
Various aldehydes and amines have been tested in order to determine whether the observed inhibition is due to a true enzyme inhibition or due to the inhibition of the reaction between thiocholine and 5,5′-dithiobis-(2-nitrobenzoic acid). 4-Dimethylaminobenzaldehyde, 3-Ethoxy-4-hydroxybenzaldehyde, diethylamine, triethylamine, triethanolamine and tyramine showed real enzyme inhibition, although their activity was about 10 3 times lower than that shown by galanthamine. Heptanal, decanal, cinnamaldehyde, anisaldehyde, benzaldehyde, hexylamine and tryptamine appeared to show a non-specific chemical inhibition.
CONCLUSIONS:
By checking this chemical inhibition on the TLC assay, the true enzyme inhibition could be distinguished from the false-positive chemical inhibition observed in the toluene extract of Nerine bowdenii in the course of isolation of active compounds.