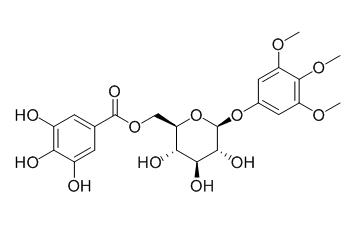
3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
3,4,5-Trimethoxyphenyl-(6'-O-galloyl)-O-beta-D-glucopyranoside shows in vitro antiplasmodial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Cell Commun Signal.2024, 22(1):597.
Indian J. of Experimental Bio.2020, 9(58).
Applied Biological Chemistry2023, 66:42.
Nutrients2020, 12(2):488
Pharmaceuticals (Basel).2024, 17(1):108.
J Appl Pharm Sci.2022, 12(04):044-053
Front Pharmacol.2021, 12:770667.
Planta Med.2023, 2192-2281
Sci Rep. 2018, 1-9
Nutrients.2020, 12(11):3448.
Related and Featured Products
Journal of Natural Products, 2001, 64(5):603.
In Vitro Antiplasmodial Activity of Extracts of Tristaniopsis Species and Identification of the Active Constituents: Ellagic Acid and 3,4,5-Trimethoxyphenyl-(6‘- O -galloyl)- O -β- d -glucopyranoside.[Reference:
WebLink]
METHODS AND RESULTS:
Screening of plants from New Caledonia for antiplasmodial activity against Plasmodium falciparum revealed that methanolic extracts of the leaves and bark of Tristaniopsis calobuxus, T. yateensis, and T.glauca inhibited the growth of chloroquine-sensitive and -resistant clones. Ellagic acid and the new compound 3,4,5-trimethoxyphenyl-(6'-O-galloyl)-O-beta-D-glucopyranoside were identified as the active constituents (IC50 0.5 and 3.2 microM, respectively). The growth inhibition of both clones was comparable.
CONCLUSIONS:
The compounds showed negligible or very low cytotoxicity to human skin fibroblasts and Hep G2 cells when tested at concentrations ranging from 0.5 to 100 microM.
Yao Xue Xue Bao, 2011, 46(8):946-950.
Phenolic acid derivatives from Bauhinia glauca subsp. pernervosa.[Pubmed:
22007520]
METHODS AND RESULTS:
To study the chemical constituents of Bauhinia glauca subsp. pernervosa, eleven phenolic acids were isolated from a 95% ethanol extract by using a combination of various chromatographic techniques including column chromatography over silica gel, ODS, MCI, Sephadex LH-20, and semi-preparative HPLC. By spectroscopic techniques including 1H NMR, 13C NMR, 2D NMR, and HR-ESI-MS, these compounds were identified as isopropyl O-beta-(6'-O-galloyl)-glucopyranoside (1), ethyl O-beta-(6'-O-galloyl)-glucopyranoside (2), 3, 4, 5-trimethoxyphenyl-(6'-O-galloyl)-O-beta-D-glucopyranoside (3),
3, 4, 5-trimethoxyphenyl-beta-D-glucopyranoside (4), gallic acid (5), methyl gallate (6), ethyl gallate (7), protocatechuic acid (8), 3, 5-dimethoxy-4-hydroxybenzoic acid (9), erigeside C (10) and glucosyringic acid (11). Among them, compound 1 is a new polyhydroxyl compound; compounds 2, 10, and 11 were isolated from the genus Bauhinia for the first time, and the other compounds were isolated from the plant for the first time.
CONCLUSIONS:
Compounds 6 and 8 showed significant protein tyrosine phosphatase1B (PTP1B) inhibitory activity in vitro with the IC50 values of 72.3 and 54.1 micromol x L(-1), respectively.