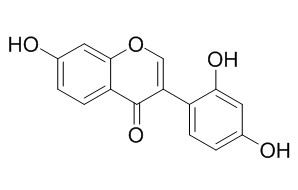
2'-Hydroxydaidzein
2'-Hydroxygenistein is an isoflavonoid phytoalexin, it suppresses chemical mediators in inflammatory cells, may have value in treatment and prevention of central and peripheral inflammatory diseases associated with excess production of chemical mediators. 2'-Hydroxygenistein shows significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B (fMLP/CB) with IC(50) values of 2.8+/-0.1 and 5.9+/-1.4 microM, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Nat Prod.2018, 81(4):966-975
Life Sci.2023, 317:121458.
Journal of Cluster Science2024, 35:635-656.
Sci Rep.2023, 13(1):13072.
The Journal of Agromedicine and Medical Sciences2018, 4(1)
Chemistry of Natural Compounds2020, 56,423-426
Carbohydrate Polymer Technologies & App.2021, 2:100049.
Heliyon.2024, 10(23):e40758.
Chem Biol Interact.2022, 368:110248.
Int J Mol Sci.2015, 16(1):1232-51
Related and Featured Products
Arch Biochem Biophys. 1990 Feb 1;276(2):390-5.
Phytoalexin synthesis in soybean: purification and characterization of NADPH:2'-hydroxydaidzein oxidoreductase from elicitor-challenged soybean cell cultures.[Pubmed:
2306102]
METHODS AND RESULTS:
An NADPH:2'-Hydroxydaidzein oxidoreductase (HDR) from elicitor-challenged soybean cell cultures was purified to apparent homogeneity by a five-step procedure. The purification procedure included affinity adsorption on Blue Sepharose and elution of the enzyme with NADP+. It was shown by gel filtration and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis that HDR consists of only one polypeptide, which has a Mr about 34,700. The pH optimum of the reaction was 7.0. Apparent Michaelis constants determined for 2'-Hydroxydaidzein, 2'-hydroxyformononetin, and NADPH were, respectively, 50, 60, and 56 microM. A low conversion of 2'-hydroxygenistein to the corresponding isoflavanone was also observed but isoflavones lacking a 2'-hydroxyl group and various other flavonoids did not serve as substrates.
CONCLUSIONS:
Enzymatically derived 2'-hydroxydihydrodaidzein gave a positive CD spectrum at 328 nm, which shows its 3R stereochemistry. Antibodies against HDR were raised in rats.
Bioorg. Med. Chem. Lett., 2004, 14(22):1011-4.
Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica.[Pubmed:
15013012]
METHODS AND RESULTS:
One new isoflavone, 5,7,4'-trihydroxy-2'-methoxyisoflavone (3) and seven, and four known compounds were isolated from the barks of Crotalaria pallida and the seeds of C. assamica, respectively.
The known compounds, apigenin (1) and 2'-hydroxygenistein (2), isolated from C. pallida, showed significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B (fMLP/CB) with IC(50) values of 2.8+/-0.1 and 17.7+/-1.9, and 5.9+/-1.4 and 9.7+/-3.5 microM, respectively. The known compounds, daidzein (4) and 2'-Hydroxydaidzein (6), isolated from C. pallida, inhibited of the release of lysozyme and beta-glucuronidase from rat neutrophils in response to fMLP/CB with IC(50) values of 26.3+/-5.5 and 13.7+/-2.6 microM, respectively. Compounds 1 and 4 also showed significant concentration-dependent inhibitory effects on superoxide anion generation in rat neutrophils stimulated with fMLP/CB with IC(50) values of 3.4+/-0.3 and 25.1+/-5.0 microM, respectively. Compounds 1 and 5, previously isolated from C. pallida, showed the inhibition of NO production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and LPS/interferon-gamma (IFN-gamma)-stimulated N9 microglial cells with IC(50) values of 10.7+/-0.1 and 13.9+/-1.1 microM, respectively.
CONCLUSIONS:
Flavonoids, suppressed chemical mediators in inflammatory cells, may have value in treatment and prevention of central and peripheral inflammatory diseases associated with excess production of chemical mediators.