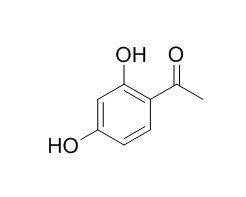
2,4-Dihydroxyacetophenone
2,4-Dihydroxyacetophenone is a plant metabolite that can act as a qualitative reagent for ferric iron.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Med Food.2019, 22(10):1067-1077
J Sep Sci.2022, 45(18):3556-3566.
United States Patent Application2020, 20200038363
Nat Commun.2025, 16(1):4121.
Cell Biochem Funct.2018, 36(6):303-311
Compounds.2023, 3(1), 169-179.
Advances in Traditional Medicine 2021, 21:779-789.
Chulalongkorn University2024, ssrn.4716057.
Antimicrob Agents Chemother.2024, e0031424.
J Ethnopharmacol.2022, 289:115018.
Related and Featured Products
Anal Sci. 2010;26(7):743-8.
MALDI mass spectrometry using 2,4,6-trihydroxyacetophenone and 2,4-dihydroxyacetophenone with cyclodextrins: suppression of matrix-related ions in low-molecular-weight region.[Pubmed:
20631433]
METHODS AND RESULTS:
Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry of a model peptide, Substance P (SubP), was carried out using 2,4,6-trihydroxyacetophenone (THAP) and 2,4-Dihydroxyacetophenone (DHAP) with cyclodextrins (cyclodextrin-supported matrix).
It was found that the use of a cyclodextrin-supported matrix simplified the mass spectrum in the low-molecular-weight region. The interaction between THAP/2,4-Dihydroxyacetophenone and cyclodextrin (CD) was studied by UV-vis absorption spectroscopy and the incorporation of matrix molecules into the cyclodextrin cavity was confirmed by (1)H-NMR spectroscopy. 2,4-Dihydroxyacetophenone showed tight incorporation with betaCD (betaCD(2,4-Dihydroxyacetophenone)) rather than THAP and it was found that the matrix-related peaks could be weakened by less than one third of the peak intensity of a protonated analyte. The betaCD(2,4-Dihydroxyacetophenone) matrix was applied to the measurements of two low-molecular-weight compounds; adenosine and adrenaline.
CONCLUSIONS:
It became clear that the cyclic structure of the CD and the host-guest interaction between betaCD and the matrix molecule were important to reduce the matrix-related peaks of THAP and 2,4-Dihydroxyacetophenone.