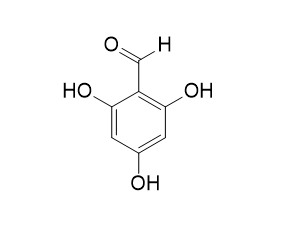
2,4,6-Trihydroxybenzaldehyde
2,4,6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice. It
is also as a potent antidiabetic agent alleviates postprandial hyperglycemia in normal and diabetic rats. 2,4,6-Trihydroxybenzaldehyde has potential anticancer activity. 2,4,6-trihydroxybenzaldehyde shows strong noncompetitive α-glucosidase inhibition (IC50 = 4.60 μM) and powerful antioxidant activity (DPPH assay) (IC50 = 71.4 μM) in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Agriculture2022, 12(2),227.
Research SPJ.2024, 0377.
J Ethnopharmacol.2024, 324:117775.
Appl. Sci.2021, 11(19),9343.
Molecules.2020, 25(23):5636.
Planta Medica International2022, 9(01):e108-e115.
PLoS One.2017, 12(3):e0173585
Food Sci Nutr.2019, 8(1):246-256
J Food Composition and Analysis2022, 104417.
Applied Biological Chem. 2020, 26(63).
Related and Featured Products
Anticancer research,2016,36(11):5743-5750.
Vanillin Analogues o-Vanillin and 2,4,6-Trihydroxybenzaldehyde Inhibit NFĸB Activation and Suppress Growth of A375 Human Melanoma.[Reference:
WebLink]
Constitutive activation of nuclear factor kappa-B (NFĸB) is a hallmark of various cancer types, including melanoma. Chemotherapy may further increase tumour NFĸB activity, a phenomenon that, in turn, exacerbates drug resistance. This study aimed at preliminary screening of a panel of aromatic aldehydes, including vanillin, for cytotoxicity and suppression of tumour cell NFĸB activity.
METHODS AND RESULTS:
The cytotoxic and NFĸB-inhibitory effects of 10 aromatic aldehydes, including vanillin, were investigated in cultured A375 human melanoma cells. Each compound was assayed alone and in combination with the model NFĸB-activating drug doxorubicin. The most promising analogues were then tested alone and in combination with 4-hydroperoxycyclophosphamide in vitro, and with cyclophosphamide in mice bearing A375 xenografts. The vanillin analogues o-vanillin and 2,4,6-Trihydroxybenzaldehyde exhibited cytotoxicity against cultured A375 cells, and inhibited doxorubicin- and 4-hydroperoxycyclophosphamide-induced NFĸB activation. They also suppressed A375 cell growth in mice.
CONCLUSIONS:
o-vanillin and 2,4,6-Trihydroxybenzaldehyde deserve further evaluation as potential anticancer drugs.
Medicinal Chemistry Research, 2010, 20(8):1181-1187.
2,4,6-Trihydroxybenzaldehyde as a potent antidiabetic agent alleviates postprandial hyperglycemia in normal and diabetic rats.[Reference:
WebLink]
METHODS AND RESULTS:
Five distinct organic compounds with protected and unprotected phenolic hydroxyl groups were screened for their α-glucosidase inhibitory potential. Of these compounds, 2,4,6-Trihydroxybenzaldehyde (THB) showed the strongest noncompetitive α-glucosidase inhibition (IC50 = 4.60 μM) and the most powerful antioxidant activity (DPPH assay) (IC50 = 71.4 μM) in vitro.
CONCLUSIONS:
In in vivo studies, THB significantly reduced blood glucose levels in normal and diabetic rats after glucose load compared to maltose load.
J Agric Food Chem, 2010, 58(9):5320-5327.
Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells.[Pubmed:
20373763]
Gut microflora metabolize anthocyanins to phenolic acids and aldehydes. These metabolites may explain the relationship between anthocyanin consumption and reduced incidence of colon cancer.
METHODS AND RESULTS:
Here, all six major metabolites, along with a Cabernet Sauvignon anthocyanin extract, were incubated with Caco-2 cells at concentrations of 0-1000 microM over 72 h to determine effects on cell proliferation and for 24 h to assess cytotoxicity effects and at 140 microM for 24 h to measure induction of apoptosis. These measurements were based on colorimetric methods. Gallic acid and 3-O-methylgallic acid inhibited cell proliferation and lacked cytotoxicity at low concentrations. The aldehyde metabolite and anthocyanin extract also inhibited cell proliferation at low concentrations and had low cytotoxicity at a wide range of concentrations. Of the four substances that effectively reduced cell proliferation, the aldehyde was the best inducer of apoptosis. In addition, these same four treatments degraded quickly in growth media, suggesting the involvement of subsequent oxidation products in the reduction of cell viability.
CONCLUSIONS:
These results indicate that the anthocyanin microfloral metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-Trihydroxybenzaldehyde reduce cell proliferation in Caco-2 cells more effectively than anthocyanins and may offer protection against colon cancer after their formation in the gut.
Environmental Toxicology and Pharmacology, 2015,39(2):962-968.
2,4,6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice .[Reference:
WebLink]
METHODS AND RESULTS:
In the present study, 2,4,6-Trihydroxybenzaldehyde (THB) was evaluated for inhibitory effects on adipocyte differentiation in 3T3-L1 cells and anti-obesity effects in mice with high-fat diet (HFD)-induced obesity. Lipid accumulation measurement indicated that THB markedly inhibited adipogenesis, and this involved down-regulation of the expression of the adipogenesis-related proteins, CCAAT/enhancer-binding protein α (C/EBPα), peroxisome proliferator-activated receptor γ (PPARγ), fatty acid synthase (FAS) and sterol regulatory element-binding protein-1c (SREBP-1c), in 3T3-L1 pre-adipocyte cells. In a mouse model of HFD-induced obesity, oral administration of THB (5 and 25 mg/kg for 13 weeks) reduced the HFD-induced increase in weight gain. THB administration also reduced serum levels of glucose, triglycerides, and total cholesterol. A reduction in the hypertrophy of white adipose tissue was also observed. Furthermore, THB administration inhibited HFD-induced hepatic steatosis.
CONCLUSIONS:
These results provided evidence that administration of THB alleviated HFD-induced obesity in C57BL/6 mice and revealed the potential of THB as a nutraceutical to help prevent or treat obesity and the associated metabolic disorders.