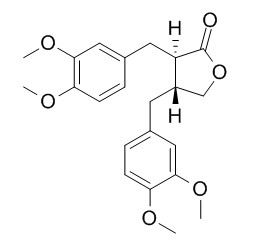
2,3-Bis(3,4-dimethoxybenzyl)butyrolactone
trans-2,3-Bis(3,4-dimethoxybenzyl)-gamma-butyrolactone is capable of inhibiting Na+,K+-ATPase activity with IC50 at a concentration less than 5 x 10(-4) M, it also shows [3H]ouabain displacement activity with IC50 at a concentration of 10(-4)-10(-3) M.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2022, 99:154025.
Foods.2020, 9(10):1348.
Nutrients.2023, 15(6):1335.
Nutrients.2018, 10(7)
VNU Journal of Science: Med.& Pharm. Sci.2022, 38(2):2588-1132.
Int J Pharm.2022, 618:121636.
J Ethnopharmacol.2018, 210:88-94
Anal Bioanal Chem.2020, 412(12):3005-3015.
Korean J. Medicinal Crop Sci.2022, 30(2):124-133
Appl Microbiol Biotechnol.2016, 100(9):3965-77
Related and Featured Products
Res Commun Chem Pathol Pharmacol. 1989 May;64(2):227-40.
Endogenous digoxin-like activity of mammalian-lignans and their derivatives.[Pubmed:
2544967]
A comparative study was made on the endogenous digoxin-like activity of sixteen mammalian-type lignan derivatives including enterolactone and enterodiol.
METHODS AND RESULTS:
Cross-reactivity to antidigoxin antibody, inhibition of dog kidney Na+,K+-ATPase, and ouabain displacing activity against [3H]ouabain binding to human erythrocytes on the part of these derivatives were examined and compared in all cases with that of ouabain. meso-2,3-Dibenzylbutane-1,4-diol (meso-HA-1) was found to possess the most potent cross-reactivity to antidigoxin antibody. Several lignans, particularly HA-1 (either meso or dl), dl-2,3-bis(3-methoxybenzyl)butane-1,4-diol(HA-4), dl-2,3-bis(3,4-dimethoxybenzyl)butane-1,4-diol(HA-10), dl-2,3-bis(3,4-dimethoxybenzyl)-1,4-dimethoxybutane(HA-11), and trans-2,3-bis(3,4-dimethoxybenzyl)-gamma-butyrolactone(HA-14) were capable of inhibiting Na+,K+-ATPase activity with IC50 at a concentration less than 5 x 10(-4) M. Meso-HA-1, HA-11 and HA-14 also showed [3H]ouabain displacement activity with IC50 at a concentration of 10(-4)-10(-3) M. These determinations of activity were made at doses 100-1000 times those of ouabain.
CONCLUSIONS:
The synthetic lignans are considered to derive in vivo from plant material usually present in fibrous food. Based on the data obtained, some lignans may possibly be endogenous digitalis-like substances.
Tetrahedron: Asymmetry.1998 August;9(16):2827–2831.
A chemoenzymatic synthesis of both enantiomers of a cis-lignan lactone.[Reference:
WebLink]
METHODS AND RESULTS:
The stereoselective acetylation of meso-2,3-bis(3,4-dimethoxybenzyl)butanediol by vinyl acetate in the presence of Candida antarctica lipase in benzene gave the corresponding (+)-(2S,3R) monoester (ee ≥98%). Transesterification of meso-2,3-bis(3,4-dimethoxybenzyl)butyl diacetate, in the presence of the same enzyme, by ethanol in benzene/isopropyl ether gave the corresponding (−)-(2R,3S) monoester (ee ≥98%).
CONCLUSIONS:
Both enantiomers of the known cis-2,3-Bis(3,4-dimethoxybenzyl)butyrolactone were synthesized from these monoesters.