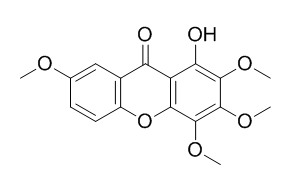
1-Hydroxy-2,3,4,7-tetramethoxyxanthone
1-Hydroxy-2,3,4,7-tetramethoxy-xanthone has vasodilatory action, it can cause vasodilation in the coronary artery pre-contracted with 1uM 5-hydroxytryptamine (5-HT), with the EC 50 value of 6.6±1.4 uM. 1-Hydroxy-2,3,4,7-tetramethoxyxanthone can effectively inhibit the osteoclast differentiation in a co-culture system with mouse osteoblastic calvarial cells and bone marrow cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Industrial Crops and Products2017, 95:286-295
Current Enzyme Inhibition2023, 19(1):55-64(10)
J Applied Biological Chemistry2021, 64(2):185-192
J Cancer.2019, 10(23):5843-5851
Foods.2023, 12(6):1227.
Food Chem.2022, 373(Pt B):131364.
Revista Brasileira de Farmacognosia2021, 31:794-804.
Pharmaceuticals (Basel).2024, 17(9):1130.
Molecules2022, 27(9):2827.
Foods.2022, 11(6):882.
Related and Featured Products
Gentisin
Catalog No: CFN89233
CAS No: 437-50-3
Price: Inquiry(manager@chemfaces.com)
Isogentisin
Catalog No: CFN70382
CAS No: 491-64-5
Price: Inquiry(manager@chemfaces.com)
1,7-Dihydroxy-4-methoxyxanthone
Catalog No: CFN89322
CAS No: 87339-76-2
Price: Inquiry(manager@chemfaces.com)
1,3,7-Trihydroxy-2-methoxyxanthone
Catalog No: CFN89266
CAS No: 211948-69-5
Price: Inquiry(manager@chemfaces.com)
1,7-Dihydroxy-2,3-dimethoxyxanthone
Catalog No: CFN89239
CAS No: 78405-33-1
Price: Inquiry(manager@chemfaces.com)
1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No: CFN89256
CAS No: 145523-71-3
Price: Inquiry(manager@chemfaces.com)
1,2,3,7-Tetramethoxyxanthone
Catalog No: CFN89234
CAS No: 22804-52-0
Price: Inquiry(manager@chemfaces.com)
1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No: CFN96239
CAS No: 14103-09-4
Price: Inquiry(manager@chemfaces.com)
1,2,3,4,7-Pentamethoxy-9H-xanthen-9-one
Catalog No: CFN98534
CAS No: 14254-96-7
Price: Inquiry(manager@chemfaces.com)
1,5-Dihydroxyxanthone
Catalog No: CFN89131
CAS No: 14686-65-8
Price: Inquiry(manager@chemfaces.com)
Phytomedicine. 2009 Dec;16(12):1144-50.
Vasodilatory actions of xanthones isolated from a Tibetan herb, Halenia elliptica.[Pubmed:
19403292 ]
In this study, six major xanthones, isolated and identified from Halenia elliptica were investigated for their vasodilatory actions in isolated rat coronary artery.
METHODS AND RESULTS:
The xanthones, including 1-hydroxy-2,3,5-trimethoxy-xanthone (HM-1), 1-hydroxy-2,3,4,7-tetramethoxy-xanthone (HM-2), 1-hydroxy-2,3,4,5-tetramethoxy-xanthone (HM-3), 1,7-dihydroxy-2,3,4,5-tetramethoxy-xanthone (HM-4), 1,5-dihydroxy-2,3-dimethoxy-xanthone (HM-5) and 1,7-dihydroxy-2,3-dimethoxy-xanthone (HM-7) caused vasodilation in the coronary artery pre-contracted with 1 microM 5-hydroxytryptamine (5-HT), with EC(50) values ranging from 1.4+/-0.1 microM (HM-1) to 6.6+/-1.4 microM (HM-2). The EC(50) values of the other xanthones were between those of HM-1 and HM-2. Removal of endothelium of the coronary artery led to decreases in the vasorelaxant effects of HM-1, HM-7 but not HM-2, HM-3, HM-4 and HM-5.
CONCLUSIONS:
Our results showed that xanthones isolated from Halenia elliptica are vasoactive substances which exhibit either endothelium-dependent or endothelium-independent mechanisms in rat coronary artery. The potency and mechanism(s) of the vasorelaxant effects of these xanthones may be relevant to the structure-activity differences in the level and the position of the substituent groups with the primary xanthone structure.
Arch Pharm Res. 2008 Jul;31(7):850-5.
Inhibitors of bone resorption from Halenia corniculata.[Pubmed:
18704326 ]
Eleven xanthones (1-11), three flavonoids (12-14) and three secoiridoids (15-17) were isolated from the aerial parts of Halenia corniculata.
METHODS AND RESULTS:
Among those compounds, 1-hydroxy-2,3,4,5-tetramethoxyxanthone (1), 1-Hydroxy-2,3,4,7-tetramethoxyxanthone (2), 1-hydroxy-2,3,4,5,7-pentamethoxyxanthone (3), 1-hydroxy-2,3,5-trimethoxyxanthone (4), 1,8-dihydroxy-3,5-dimethoxyxanthone (7), and luteolin (12), at the concentration of 1 microg/mL, effectively inhibited the osteoclast differentiation in a co-culture system with mouse osteoblastic calvarial cells and bone marrow cells. Notably, compounds 1, 3, and 4 exhibited, in a dose-dependent manner, significant inhibition of osteoclast differentiation even at a low concentration (0.01 microg/mL). All the inhibitory compounds, except for compound 7, significantly reduced the pit formation on the dentine slice compared with the control group. For the survival of the mature osteoclasts, compounds 1-4 and 12 (1 microg/mL), significantly decreased the survival number through induction of cell apoptosis, and compound 4 exhibited a significant inhibitory effect on osteoclast survival even at the low concentration of 0.1 microg/mL.