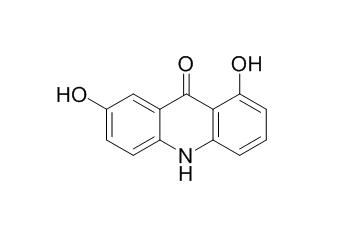
1,7-Dihydroxyacridone
1,7-Dihydroxyacridone is a natural product from Boenninghausenia albiflora.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Integrative Medicine Research2024, 13(1):101025.
LWT2020, 130:109535
Crystals2020, 10(3), 206.
Malaysian Journal of Analytical Sciences2023, 27(4):840-848.
Cancers (Basel).2021, 13(9):2223.
Front Microbiol.2022, 13:835463.
Front Microbiol.2021, 12:736780.
Molecules.2016, 21(10)
Eur J Pharmacol.2024, 963:176280.
Onco Targets Ther.2017, 10:3467-3474
Related and Featured Products
Supplements to Edition of Rodds Chemistry of Carbon Compounds, 1975:245-258.
Sainsbury M. Chapter 32–The acridine alkaloids.[Reference:
WebLink]
This chapter provides an overview of the acridine alkaloids. Gravacridone triol represents a new alkaloid from Ruta graveolens, where it co-occurs with its monoglucosyl derivative and the glucoside of a known alkaloid gravacridone diol.
METHODS AND RESULTS:
The 5-hydroxy derivative of the known alkaloid arborinine has been obtained for the first time from the leaves of Glycosmis bilocularis. Two other acridones differing only in the presence or absence of methoxyl groups are the alkaloids and isolated from the leaves of Bauerella simplicifolia. Boenninghausenia albiflora produces 1-hydroxyacridone and possibly also 1,7-Dihydroxyacridone.
CONCLUSIONS:
Two new acridones, 1-hydroxy-3,4-dimethoxy-10-methylacridone and l-hydroxy-3-geranyloxy-4-methoxy-10-methylacridone occur in extracts of the plant Sarcomelicope leiocarpa indigenous to New Caledonia together with eight other known structures. Another series of related alkaloids are produced by the plant Glycosmis citrifolia; these include the acridones glycocitrine-I, glycocitrine-II, its o-methyl ether, glyfoline, furofoline-II, and pyranofoline.