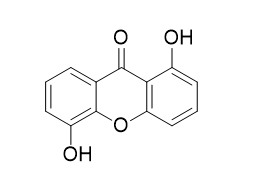
1,5-Dihydroxyxanthone
1,5-Dihydroxyxanthone exhibits the epidermal growth factor receptor (EGFR) -tyrosine kinase inhibitory activity, with the IC50 value of 90.34 nM. It may have anticholinesterase activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean J of Crop Science2019, 452-458
Journal of Oil Palm Research2019, 31(2):238-247
J Ethnopharmacol.2024, 318:116863.
J Drug Delivery Science and Tech.2022, 67:102957.
J Appl Microbiol.2022, 132(2):949-963.
FEBS Lett.2021, 595(20):2608-2615.
Biomed Pharmacother.2019, 111:262-269
Nutrients.2024, 16(22):3805.
Pharmaceutics.2023, 15(9):2355.
Food Chem. 2020, 320:126530
Related and Featured Products
Kielcorin
Catalog No: CFN89359
CAS No: 64280-48-4
Price: Inquiry(manager@chemfaces.com)
Cadensin D
Catalog No: CFN96957
CAS No: 102349-35-9
Price: Inquiry(manager@chemfaces.com)
Euxanthone
Catalog No: CFN98879
CAS No: 529-61-3
Price: Inquiry(manager@chemfaces.com)
Gentisin
Catalog No: CFN89233
CAS No: 437-50-3
Price: Inquiry(manager@chemfaces.com)
Isogentisin
Catalog No: CFN70382
CAS No: 491-64-5
Price: Inquiry(manager@chemfaces.com)
1,7-Dihydroxy-4-methoxyxanthone
Catalog No: CFN89322
CAS No: 87339-76-2
Price: Inquiry(manager@chemfaces.com)
1,3,7-Trihydroxy-2-methoxyxanthone
Catalog No: CFN89266
CAS No: 211948-69-5
Price: Inquiry(manager@chemfaces.com)
1,7-Dihydroxy-2,3-dimethoxyxanthone
Catalog No: CFN89239
CAS No: 78405-33-1
Price: Inquiry(manager@chemfaces.com)
1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No: CFN89256
CAS No: 145523-71-3
Price: Inquiry(manager@chemfaces.com)
1,2,3,7-Tetramethoxyxanthone
Catalog No: CFN89234
CAS No: 22804-52-0
Price: Inquiry(manager@chemfaces.com)
Phytochemistry. 2008 Feb;69(4):1013-7.
Polyanxanthone A, B and C, three xanthones from the wood trunk of Garcinia polyantha Oliv.[Pubmed:
18022654]
METHODS AND RESULTS:
Three xanthones, polyanxanthone A (1), B (2) and C (3) have been isolated from the methanol extract of the wood trunk of Garcinia polyantha, along with five known xanthones: 1,3,5-trihydroxyxanthone (4); 1,5-Dihydroxyxanthone (5); 1,3,6,7-tetrahydroxyxanthone (6); 1,6-dihydroxy-5-methoxyxanthone (7) and 1,3,5,6-tetrahydroxyxanthone (8). Their structures were determined by means of 1D- and 2D-NMR techniques.
CONCLUSIONS:
Some of the above compounds were screened for their anticholinesterase activity on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes.
Molecules. 2014 Nov 28;19(12):19923-34.
Antibacterial and EGFR-tyrosine kinase inhibitory activities of polyhydroxylated xanthones from Garcinia succifolia.[Pubmed:
25460314 ]
METHODS AND RESULTS:
Chemical investigation of the methanol extract of the wood of Garcinia succifolia Kurz (Clusiaceae) led to the isolation of 1,5-Dihydroxyxanthone (1), 1,7-dihydroxyxanthone (2), 1,3,7-trihydroxyxanthone (3), 1,5,6-trihydroxyxanthone (4), 1,6,7-trihydroxyxanthone (5), and 1,3,6,7-tetrahydroxyxanthone (6). All of the isolated xanthones were evaluated for their antibacterial activity against bacterial reference strains, two Gram-positive (Staphylococcus aureus ATTC 25923, Bacillus subtillis ATCC 6633) and two Gram-negative (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853), and environmental drug-resistant isolates (S. aureus B1, Enteroccoccus faecalis W1, and E. coli G1), as well as for their epidermal growth factor receptor (EGFR) of tyrosine kinase inhibitory activity.
CONCLUSIONS:
Only 1,5,6-trihydroxy-(4), 1,6,7-trihydroxy-(5), and 1,3,6,7-tetrahydroxyxanthones (6) exhibited antibacterial activity against Gram-positive bacteria, however none was active against vancomycin-resistant E. faecalis. Additionally, 1,7-dihydroxyxanthone (2) showed synergism with oxacillin, but not with ampicillin. On the other hand, only 1,5-Dihydroxyxanthone (1) and 1,7-dihydroxyxanthone (2) were found to exhibit the EGFR-tyrosine kinase inhibitory activity, with IC50 values of 90.34 and 223 nM, respectively.
Nat Prod Res. 2017 Nov;31(21):2513-2519.
A new pyranoxanthone from Garcinia nervosa.[Pubmed:
28412841 ]
METHODS AND RESULTS:
Phytochemical studies on the stem bark of Garcinia nervosa has resulted in the discovery of one new pyranoxanthone derivative, garner xanthone (1) and five other compounds, 1,5-Dihydroxyxanthone (2), 6-deoxyisojacareubin (3), 12b-hydroxy-des-D-garcigerrin A (4) stigmasterol (5), and β-sitosterol (6). The structures of these compounds were elucidated with the aid of spectroscopic techniques, such as NMR and MS.
CONCLUSIONS:
The crude extracts of the plant were assessed for their antimicrobial activity.