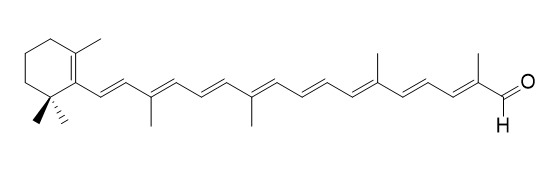
trans-Beta-Apo-8'-carotenal
beta-Apo-8'-carotenal, canthaxanthin and astaxanthin, are inducers of CYP1A1 and 1A2 in the rat. These carotenoids form a new class of inducers of CYP1A, structurally very different from the classical inducers as 3-methylcholanthrene, beta-naphtoflavone or dioxin.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2020, 249:112396
Pharmaceuticals (Basel).2024, 17(4):442.
Front Plant Sci.2022, 13: 905275.
Toxicol Res.2019, 35(4):371-387
Front. Pharmacol.2022, 901563.
J of l. Chroma.&Related Tech2020, 43(11-12):414-423.
PLoS One.2018, 13(4):e0195642
Int J Biol Macromol.2020, 161:1230-1239.
Pharmaceutics.2023, 15(9):2355.
Phytomedicine.2022, 100:154036.
Related and Featured Products
Xenobiotica, 1996, 26(9):909-919.
beta-Apo-8'-carotenal, but not beta-carotene, is a strong inducer of liver cytochromes P4501A1 and 1A2 in rat.[Pubmed:
8893038]
METHODS AND RESULTS:
1. The catalytic activities of several phase I and II xenobiotic-metabolizing enzymes and their immunochemical detection have been investigated in liver microsomes and cytosol of the male rat, which had been fed for 15 days with diets containing 300 mg/kg beta-carotene isomers (all-trans beta-carotene or beta-carotene from Dunaliella salina rich in 9-cis isomer or isomerized beta-carotene), or apocarotenoids as beta-apo-8'-carotenal, ethyl beta-apo-8'-carotenoate and citranaxanthin. 2. Beta-carotene, either all-trans or containing cis isomers, did not induce any significant change in the measured activities. By contrast, beta-apo-8'-carotenal increased the liver content of cytochrome P450, the activity of NADH- and NADPH-cytochrome c reductase, and strongly increased some cytochrome P450-dependent activities, particularly ethoxyresorufin O-deethylase (x158), methoxyresorufin O-demethylase (x22), pentoxy- and benzoxyresorufin O-dealkylases, but did not affect erythromycin N-demethylase nor nitrosodimethylamine N-demethylase activities. Phase II p-nitrophenol- and 4-hydroxy- biphenyl-uridine diphosphoglucuronosyl transferase activities were also increased by beta-apo-8'carotenal. Western blots of microsomal proteins clearly showed the induction of CYP1A1 and 1A2 by beta-apo-8'-carotenal. This induction profile resembles that produced by two other carotenoids: canthaxanthin and astaxanthin. Ethyl beta-apo-8'-carotenoate and citranaxanthin showed similar effects to beta-apo-8'-carotenal but of less intensity.
CONCLUSIONS:
3. Three carotenoids: beta-apo-8'-carotenal, canthaxanthin and astaxanthin, are inducers of CYP1A1 and 1A2 in the rat. These carotenoids form a new class of inducers of CYP1A, structurally very different from the classical inducers as 3-methylcholanthrene, beta-naphtoflavone or dioxin.
Proc Spie, 1995, 2362:231-239.
Photoconductivity of all trans-beta-carotene and all-trans-beta-apo-8'-carotenal crystals.[Reference:
WebLink]
METHODS AND RESULTS:
Three different kinds of condensed samples of all-trans-(beta) -carotene, i.e., a spin-coated film, a cast film, and crystals, were prepared. Excitation spectra of photoconductivity at room temperature and resonance Raman scattering at 11 K were obtained for these samples, and the influence of oxygen to these excitation spectra were examined. In the near infrared region below the lowest allowed transition to the electronically excited 1Bu state, clear bands were observed in those two kinds of excitation spectra and were characteristic of the crystals as well as the cast film. The excitation bands are attributable to the same transition to the 21Ag molecular exciton state. A similar series of experiments were carried out for
all-trans-Beta-Apo-8'-carotenal .
CONCLUSIONS:
The results support the above assignment for all-trans- (beta) -carotene.