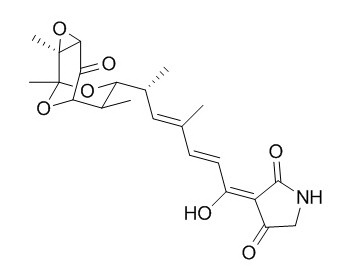
Tirandamycin A
Tirandamycin A and echinomycin A have anti-amoebic properties, they inhibit the in vitro growth of a E. histolytica laboratory strain (HM-1:IMSS) and a clinical isolate (Colombia, Col) at 30- to 60- uM concentrations. Tirandamycin A and streptolydigin possess potent antibacterial activity particularly against anaerobes, have been shown to inhibit bacterial RNA polymerase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2021, 26(12):3652.
J Hematol Oncol.2018, 11(1):112
Molecules.2020, 25(21):5087.
Plant Methods.2017, 13:108
Phytother Res.2016, 30(12):2020-2026
Medicinal Chemistry Research 2021, 30:1117-1124.
UDC.2020, 19(4).
J Med Food.2024, 27(9):844-856.
Pharmacognosy Magazine2018, 14(56):418-424
J Biochem.2024, 175(3):253-263.
Related and Featured Products
Parasitol Res. 2012 Dec;111(6):2473-7.
Antiamoebic properties of the actinomycete metabolites echinomycin A and tirandamycin A.[Pubmed:
22763704]
Entamoeba histolytica infects 50 million people per year, causing 100,000 deaths worldwide. The primary treatment for amoebiasis is metronidazole. However, increased pathogen resistance combined with the drug's toxic side effects encourages a search for alternative therapeutic agents.
METHODS AND RESULTS:
Secondary metabolites from marine bacteria are a promising resource for antiprotozoan drug discovery. In this study, extracts from a collection of marine-derived actinomycetes were screened for antiamoebic properties, and the activities of antibiotics echinomycin A and Tirandamycin A are shown.
CONCLUSIONS:
Both antibiotics inhibited the in vitro growth of a E. histolytica laboratory strain (HM-1:IMSS) and a clinical isolate (Colombia, Col) at 30- to 60-μM concentrations. EIC(50) (estimated inhibitory concentration) values were comparable for both antibiotics (44.3-46.3 μM) against the E. histolytica clinical isolate.
Tetrahedron Letters,1991,32(14):1749–1752.
Asymmetric aldol reactions using chiral boron reagents: Application to the synthesis of tirandamycin A[Reference:
WebLink]
METHODS AND RESULTS:
The bicyclic acetal 1, a key intermediate in previous synthetic studies on Tirandamycin A, has been prepared in enantiomerically pure form starting from the (R)-ethylketone 2 and the aldehyde 3. A reagent controlled aldol reaction using (-)-(Ipc)2BOTf selectively gave the 1,2-syn–2,4-syn adduct 4, which was subsequently converted into 1via the 1,3-anti diol 5.
Journal of Medicinal Chemistry,1989 ,32 (5) :1062-9
Aromatic dienoyl tetramic acids. Novel antibacterial agents with activity against anaerobes and staphylococci[Reference:
WebLink]
Streptolydigin (1) and Tirandamycin A (2) are typical members of the naturally occurring class of 3-dienoyl tetramic acids. These compounds, which possess potent antibacterial activity particularly against anaerobes, have been shown to inhibit bacterial RNA polymerase. In contrast, tenuazonic acid (5), which lacks a complex dioxabicyclononane moiety and diene chromophore present in 1 and 2, exhibits essentially no antimicrobial activity and has no effect on bacterial RNA polymerase, suggesting that one or both of these structural features may be critical for antibacterial activity.
METHODS AND RESULTS:
In this paper, we report on a novel series of synthetic dienoyl tetramic acids that lack a complex dioxabicyclononane unit. Several of these compounds, particularly 8T-W, exhibit potent antimicrobial activity against Gram-positive and Gram-negative anaerobes as well as staphylococci. We will discuss the structure-activity relationship for this series of compounds which, in contrast to their natural counterparts, do not inhibit significantly RNA polymerase. We will also discuss preliminary results on the biochemical and microbiological properties of this series of compounds, several of which moderately inhibit supercoiling by DNA gyrase isolated from E. coli H560, although this enzyme has not been established as their target in whole cells.
CONCLUSIONS:
Compound 8W, which is not cross-resistant with DNA gyrase subunit A or B inhibitors or tirandamycin, has also been demonstrated to be rapidly bactericidal.