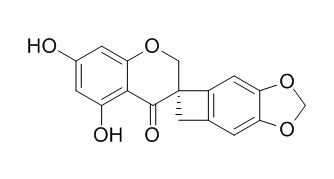
Scillascillin
Scillascillin has anticancer activity, it is significantly active against human cancer cell lines MCF-7 (breast cancer) and DU-145 (prostate cancer) with inhibitory concentration (IC)50 values 9.59 and 11.32 ug/ml respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Appl. Sci. 2024, 14(13), 5815
Biochem Biophys Res Commun.2018, 505(1):261-266
Biochem Pharmacol. 2023, 210:115463.
Analytical Methods2018, 10(27)
Int J Mol Sci.2022, 23(11):6104.
J Microbiol Biotechnol.2023, 33(10):1317-1328.
Korean Journal of Pharmacognosy2014, 113-120
Molecules.2019, 24(1):E159
Biomed Pharmacother.2024, 175:116770.
Int Immunopharmacol. 2020, 83:106403.
Related and Featured Products
Pharmacognosy Res. 2014 Oct;6(4):303-5.
Anticancer Active Homoisoflavone from the Underground Bulbs of Ledebouria hyderabadensis.[Pubmed:
25276067]
Ledebouria is a genus of deciduous or weakly evergreen bulbs in the Hyacinthaceae family. This is recognized as the first collection made of the new taxon Ledebouria hyderabadensis, exist in the Hyderabad city of Andhra Pradesh, India.
The goal of this work was to investigate the phytochemical constituents present in the new specifies and also to evaluate the cytotoxic properties of the extracts and pure compounds against human cancer cell lines.
METHODS AND RESULTS:
The anticancer activity was evaluated in in vitro mode by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test.
Phytochemical investigation of underground bulbs of indigenous, rare, and recently identified herb L. hyderabadensis yielded a bioactive homoisoflavanone, Scillascillin 1. The structure of the compound was established on the basis of various nuclear magnetic resonance and mass spectral data. The compound Scillascillin was isolated for the first time from L. hyderabadensis.
CONCLUSIONS:
In vitro anticancer activity, performed using MTT assay, showed compound 1 as significantly active against human cancer cell lines MCF-7 (breast cancer) and DU-145 (prostate cancer) with inhibitory concentration (IC)50 values 9.59 and 11.32 μg/ml respectively when compared with herb methanol extract (IC50 values 36.21 and 44.86 μg/ml respectively).
Zhongguo Zhong Yao Za Zhi. 2014 Oct;39(19):3788-93.
Homoisoflavanones and stilbenes from fresh bulb of Scilla scilloides.[Pubmed:
25612441]
Mian-Zao-Er was collected from the bulbs of Scilla scilloides (Lindl. ) Druce, belonging to the Hyacinthaceae family.
METHODS AND RESULTS:
17 compounds were obtained using various column chromatographies on macroporus resin (HPD100), silica gel, Sephadex LH-20 and ODS, as well as semi-preparative HPLC. Their structures were elucidated on the basis of physicochemical properties and spectral data as 2-hydroxy-7-methoxyScillascillin (1), Scillascillin (2), 5,7-dihydroxy-3',4'-dimethoxyspiro 2H-1-benzopyran-7'-bicyclo[4.2.0 ] octa [1,3,5 ] -trien } -4-one (3), socialinone (4), 4-methylresveratrol (5), (E)-resveratrol (6), scillavoneA (7), 3,9-di- hydroeucomnalin (8), 3-(3-hydroxy-4-methoxybenzyl) -5,7-dihydroxychroman-4-one (9), (3R)-5,7,3'-trihydroxy-4'-methoxyspiro (2H-1-benzopyran-7'-bicyclo[4, 2, 0] octa [1, 3, 5]-trien} -4-one (10), scillabene A (11), 2-hydroxyScillascillin (12), 3-(4-hydroxybenzyl) -5,7-dihydroxychroman-4-one (13), 3-( 4-hydroxybenzylidene) -5, 7-dihydroxychroman-4-one (14), 3-( 4-hydroxybenzyl) -5-hydroxy-7,8-dimethoxychroman-4-one (15), 3-(4-hydroxybenzyl) -5-hydroxy-6, 7-dimethoxychroman-4-one (16), and 3-(4-hydroxybenzyl)-5,8-hydroxy-7-methoxychroman-4-one (17).
METHODS AND RESULTS:
Among them, compounds 3, 4, 6, 9, 13 and 15-17 were isolated from this plant for the first time.