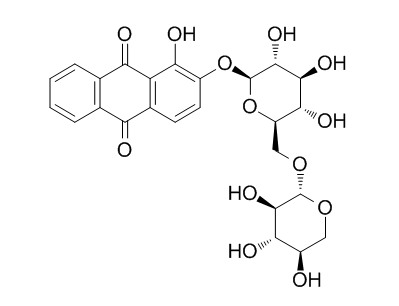
Ruberythric acid
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2021, 12:615157.
Metabolites. 2023, 13(11):1122.
Mediators Inflamm.2016, 2016:7216912
Journal of Chromatography A2020, 460942
Internoational J of Toxicology2020, 10.1177.
Exp Ther Med.2019, 18(6):4388-4396
Molecules.2019, 24(17):E3127
Int J Anal Chem.2017, 2017:1254721
Phytomedicine Plus2022, 2(1):100207.
Sci Rep. 2017, 8207(7)
Related and Featured Products
Phytochemistry,2015,117:168-173.
Isolation and extraction of ruberythric acid from Rubia tinctorum L. and crystal structure elucidation.[Reference:
WebLink]
Madder (Rubia tinctorum L.) has been exploited as a dye throughout history.
METHODS AND RESULTS:
The roots of the plant are very rich in the highly coloured glycosidic compounds Ruberythric acid and lucidin primeveroside, alongside the corresponding aglycons which can be readily formed by deglycosylation, particularly during extraction.
CONCLUSIONS:
Supported by 1H and 13C NMR data, the conclusive X-ray crystal structure of the natural dye Ruberythric acid is presented for the first time. The solid state structure revealed extensive intermolecular hydrogen bonding interactions between the sugar moieties in the unit cell, but only intramolecular hydrogen bonding through the hydroxyquinone groups. There is also some additional π–π stacking from the anthraquinone moiety.