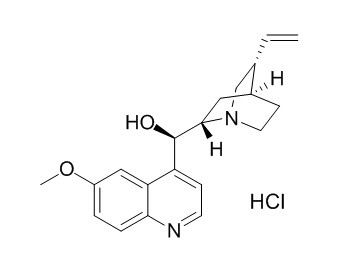
Quinine HCl
Quinine HCl produces alpha-adrenergic blockade. Quinine modifies catecholamine- and calcium-induced myocardial contractile force responses.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Microbiol Biotechnol.2023, 33(10):1317-1328.
Food Funct.2021, 12(4):1469-1481.
Environ Toxicol.2023, 23929.
Chemistry of Natural Compounds2020, 56,423-426
Molecules.2016, 21(6)
VNU Journal of Science2023, 39(2):24-33.
J Cell Mol Med.2023, 27(11):1592-1602.
Phytomedicine.2017, 24:77-86
Molecules.2023, 28(8):3376.
Biomed Pharmacother.2024, 175:116770.
Related and Featured Products
Zoolog Sci. 1995 Feb;12(1):45-52.
Quinine HCl-induced modification of receptor potentials for taste stimuli in frog taste cells.[Pubmed:
7795491]
METHODS AND RESULTS:
After frog taste cells were adapted to 1 mM Quinine HCl (Q-HCl) for 10 sec, modification of receptor potentials in the taste cells induced by salt, acid, sugar and bitter stimuli was studied with microelectrodes. The phasic component of receptor potentials induced by 0.1 M NaCl, KCl, NH4Cl and MgCl2 was enhanced following adaptation to Quinine HCl. The rate of rise of receptor potentials in response to the salts was increased after Quinine HCl adaptation. The amplitude and the rate of rise of receptor potentials induced by 1 mM acetic acid were larger after Quinine HCl adaptation than after water adaptation. The amplitude of phasic component and rate of rise of receptor potentials for 0.5 M sucrose after Quinine HCl were the same as those after water. The amplitudes of tonic receptor potentials for 1 mM Q-H2SO4, brucine and picric acid after Quinine HCl adaptation were the same as those after 1 mM NaCl adaptation. Correlation coefficient between taste cell responses induced by 1 mM Quinine HCl and 1 mM Q-H2SO4 was very high, but those between 1 mM Quinine HCl and 1 mM brucine responses and between 1 mM Quinine HCl and 1 mM picric acid responses were low.
CONCLUSIONS:
This indicates that Quinine HCl and Q-H2SO4 bind to the same receptor site, but brucine and picric acid bind to different receptor sites to which Quinine HCl does not bind.
Eur J Pharmacol. 1980 May 2;63(2-3):159-66.
alpha-Adrenergic blocking properties of quinine HCl.[Pubmed:
6103815]
METHODS AND RESULTS:
In the anesthetized dog, Quinine HCl (50 mg/kg, i.v.) infused over a 20 min period produced 1 22% maximum decrease in diastolic blood pressure, a 53% increase in pulse pressure and a 52% increase in myocardial contractile force. The initial positive inotropic response was maximal in the first 5--15 min of the quinine infusion and decreased to near control levels 40 min following the quinine infusion. Quinine caused a marked reduction in the noradrenaline (NA) pressor response, blockade of the adrenaline (A) pressor response, partial blunting of the angiotensin II (AII) pressor effect but no change in the depressor effect of isoprenaline (I). The positive inotropic effects of CaCl2 were reduced and the duration of contractile action to both I and CaCl2 was significantly prolonged by quinine. In isolated rabbit thoracic aortic strips, quinine produced a parallel, dose-related shift of the concentration-response curve for NA to the right but did not affect the maximum responses. A pA2 of 4.91 was estimated by the method of Schild. The determined line had a slope of -0.84 which is similar to a theoretical slope of -1.0 and indicates a direct relationship between the number of receptors occupied and the contractile response. The responses to AII and histamine (H) were not altered by quinine.
CONCLUSIONS:
These results suggest that Quinine HCl produces alpha-adrenergic blockade; additionally, quinine modifies catecholamine- and calcium-induced myocardial contractile force responses.
Hum Mol Genet. 2010 Nov 1;19(21):4278-85.
The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12.[Pubmed:
20675712]
METHODS AND RESULTS:
The perceived taste intensities of Quinine HCl, caffeine, sucrose octaacetate (SOA) and propylthiouracil (PROP) solutions were examined in 1457 twins and their siblings. Previous heritability modeling of these bitter stimuli indicated a common genetic factor for quinine, caffeine and SOA (22-28%), as well as separate specific genetic factors for PROP (72%) and quinine (15%). To identify the genes involved, we performed a genome-wide association study with the same sample as the modeling analysis, genotyped for approximately 610,000 single-nucleotide polymorphisms (SNPs). For caffeine and SOA, no SNP association reached a genome-wide statistical criterion. For PROP, the peak association was within TAS2R38 (rs713598, A49P, P = 1.6 × 10(-104)), which accounted for 45.9% of the trait variance. For quinine, the peak association was centered in a region that contains bitter receptor as well as salivary protein genes and explained 5.8% of the trait variance (TAS2R19, rs10772420, R299C, P = 1.8 × 10(-15)). We confirmed this association in a replication sample of twins of similar ancestry (P = 0.00001).
CONCLUSIONS:
The specific genetic factor for the perceived intensity of PROP was identified as the gene previously implicated in this trait (TAS2R38). For quinine, one or more bitter receptor or salivary proline-rich protein genes on chromosome 12 have alleles which affect its perception but tight linkage among very similar genes precludes the identification of a single causal genetic variant.