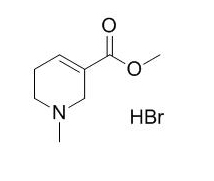
Arecoline hydrobromide
Arecoline hydrobromide is formerly used as a cholinergic agent, it is a hypotensive agent. Arecoline hydrobromide is purgative, vermifuge and taenifuge agents in veterinary medicine. It induces rat hepatic CYP2 B by promoting nuclear translocation of CAR, the regulation of it on rat hepatic CYP2 B largely involve transcriptional activation of the gene,partially involve the post-translational modification of CYP2 B protein.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Nat Med.2022, 76(1):59-67.
Vojnosanit Pregl2016, 75(00):391-391
Nat Commun.2023, 14(1):5075.
Plants (Basel).2023, 12(22):3877.
J Ethnopharmacol.2020, 269:113752.
Food Chem.2024, 460(Pt 1):140472.
Asian J Beauty Cosmetol2016, 14(3):249-257
Life Sci.2023, 332:122107.
Applied Biological Chemistry 2021, 64(75)
Geroscience.2024, 01207-y.
Related and Featured Products
Animal Husbandry and Feed Science, 2010(z1):30-32.
Effects of arecoline hydrobromide on taeniasis and cysticercosis in domestic animals.[Reference:
WebLink]
METHODS AND RESULTS:
Taeniasis and cysticercosis in domestic animals belong to zoonosis and seriously threaten the public health security.Especially the cysticercosis and echinococcosis caused by the tapeworm eggs have great harms to bodies because they can attack many organs of body.According to the combination of experimental results and literature materials,the morphology and transmission mode of taenia and cysticercus,the prevalence status and monitoring of taeniasis and cysticercosis as well as the antitapeworm mechanism,comparative analysis to other drugs,expelling tapeworm tests in vitro,dose determining tests and usage notes of Arecoline hydrobromide were expounded in detail.
CONCLUSIONS:
It provides a theoretical basis for prevention of taeniasis and cysticercosis and more scientific usage of Arecoline hydrobromide and thus relieves the harms of taeniasis and cysticercosis and ensuring the public health security.
Chinese Traditional & Herbal Drugs, 2016.
In vivo effect of arecoline hydrobromide on rat hepatic CYP2B expression and its mechanism.[Reference:
WebLink]
To study the effect of Arecoline hydrobromide(AH) on rat hepatic CYP2 B expression/activity,as well as the underlying regulation mechanism in vivo.
METHODS AND RESULTS:
After oral administration of AH(4,20,and 100 mg/kg/d) to rats for 7 consecutive days,the hepatic CYP2 B activity was detected by LC-MS/MS method,the protein levels of hepatic CYP2 B,total CAR,and endonuclear CAR were detected by Western blotting,and the hepatic CYP2B1 mRNA level was detected by real-time PCR. AH treatment had no effect on rat hepatic CYP2 B protein level,but the hepatic CYP2B1 mRNA level was dose-dependency increased.Additionally,although the hepatic CYP2 B activity was induced by AH treatment,the induction was weakened with the dose increase of AH.Furthermore,the protein content of hepatic endonuclear CAR was increased while the total CAR protein remained unchanged following AH treatment.
CONCLUSIONS:
AH induces rat hepatic CYP2 B by promoting nuclear translocation of CAR.The regulation of AH on rat hepatic CYP2 B largely involve transcriptional activation of the gene,partially involve the post-translational modification of CYP2 B protein.
Our results also suggest that the risk of metabolic interaction could be existed when the substrate drugs of CYP2 B are administered in betel-quid used human.