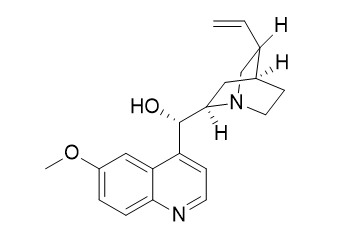
(+)-Quinidine
Quinidine, a useful antiarrhythmic compound, is usually contaminated with dihydroquinidine, a compound that itself shows potent antiarrhythmic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
PLoS One.2018, 13(3):e0193386
Front Microbiol.2022, 13:835463.
Molecules.2019, 24(2):329
Process Biochemistry2019, 87:213-220
Eur J Ther.2023, 29(4):900-906.
Plant Physiol.2024, 194(4):2580-2599.
Environ Toxicol.2023, tox.23999.
Biomed Pharmacother.2022, 146:112497.
Buildings2023, 13(5), 1112.
J Chromatogr B Analyt Technol Biomed Life Sci.2019, 1124:323-330
Related and Featured Products
Chemical Physics Letters, 1994, 222(4):403-410.
Isolation of preresonance and out-of-phase dual circular polarization Raman optical activity.[Reference:
WebLink]
Quinidine, a useful antiarrhythmic compound, is usually contaminated with dihydroquinidine, a compound that itself shows potent antiarrhythmic activity.
METHODS AND RESULTS:
Complete hydrogenation of quinidine followed by conversion to dihydroquinidine derivatives was explored as a basis for eliminating the analytical problems inherent in the quality control of quinidine products and for determining their pharmacological potency and pharmacokinetic parameters without interfering impurities. Attempts to resolve the quinidine analogs, dihydrocupreidine, and its benzoyloxy ester failed with normal and reversed-phase chromatography. Ion-pairing chromatography using n-octanesulfonate in methanol:water proved successful.
CONCLUSIONS:
Using 9-hydroxy-4-methoxy acridine as internal standard, separation and quantitation of the dihydroquinidine analogs from spiked plasma samples was achieved with 92 to 95% efficiency.