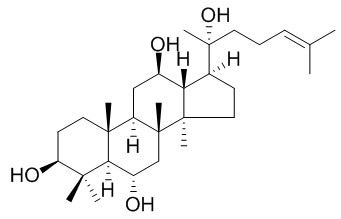
20(R)-Protopanaxatriol
20(R)-Protopanaxatriol shows anti-hyperglycaemic, and anti-cancer activities.Protopanaxatriol has inhibitory effects on the enzyme catalytic activities of cyclooxygenases-1 and -2 (COX-1 and -2).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
BMC Complement Altern Med.2019, 19(1):339
Mol Plant Pathol.2022, 10.1111:mpp.13280.
Earth Environ. Sci. 2021, 905:012080.
Drug Dev Ind Pharm.2024, 50(11):938-951.
Nutrients.2023, 15(12):2810.
J AOAC Int.2023, 106(1):56-64.
Asian Journal of Chemistry2014, 26(22):7811-7816
Agronomy2020, 10(3),388.
Cancers (Basel).2021, 13(17):4327.
Molecules 2022, 27(3),960.
Related and Featured Products
Food Chem., 2012, 133(3):998-1000.
Cyclooxygenase inhibitory activity of ginsenosides from heat-processed ginseng.[Reference:
WebLink]
Ginsenosides, from heat-processed ginseng, and sapogenins were evaluated for their inhibitory effects on the enzyme catalytic activities of cyclooxygenases-1 and -2 (COX-1 and -2).
METHODS AND RESULTS:
The ginsenosides, 20(S)-Rg3, Rg5, and Rk1, inhibited COX-2 activity, but did not affect the enzyme activity of COX-1. Protopanaxatriol (PPT) moderately inhibited both COX-1 and -2. The ginsenosides, 20(R)-Rg3, Re, and protopanaxadiol (PPD) showed a minimal effect on COX-1 and -2 activities.
CONCLUSIONS:
Taken together, ginsenosides Rg3 (20S-form), Rg5 and Rk1 showed selective inhibitory activity on COX-2 by behaving as an inhibitor of the enzyme–substrate reaction.
Chemical Research in Chinese Universities, 2016, 32(1): 1-6.
Semisynthesis and cytotoxicity evaluation of a series of ocotillol type saponins and aglycones from 20(S)-ginsenoside Rg2, Rh1, protopanaxatriol and their 20(R)-epimers[Reference:
WebLink]
With the oxidation treatment, eighteen compounds were separated from 20(S)-ginsenoside Rg2, Rh1, protopanaxatriol(PPT) and their 20(R)-epimers in total and cytotoxicity of most of them was evaluated against three human cancer cell lines HeLa, A549 and MCF-7 by 3-(4,5-dimetylthiazol-z-yl)-2,5-diphenyltetrazolium bromide(MTT) assay.
METHODS AND RESULTS:
Their structures were confirmed by means of nuclear magnetic resonance(NMR) and mass spectrometry and the results were compared with those of previous literature. In this study, we systematically semisynthesized all four ocotillol type saponins, i.e., (20S, 24S), (20S, 24R), (20R, 24S) and (20R, 24R). All the configurations at C20 kept the same with their starting materials. Meanwhile a pair of C24 epimers was generated in terms of ocotillol type saponins. In addition, seven compounds(4―8, 13 and 14) were reported firstly.
CONCLUSIONS:
The cytotoxic results distinguished the ocotillol type products(6, 7, 13 and 14) from 20(R)-Protopanaxatriol and 20(R)-ginsenoside Rh1, which possessed better cytotoxicities than their correspondents from 20(S)-epimers against HeLa cells, and the carbonyl group at C3 can improve the cytotoxicity, which helped us to gain deeper insight into Ocotillol type saponins.
J. Funct. Foods, 2016, 23:188-97.
The inhibition of α-glycosidase and protein tyrosine phosphatase 1B (PTP1B) activities by ginsenosides from Panax ginseng C.A. Meyer and simultaneous determination by HPLC-ELSD.[Reference:
WebLink]
Panax ginseng C.A. Meyer is extensively used as a food additive because of its medicinal and nutritional properties.
METHODS AND RESULTS:
This study investigated the inhibitory activity of eight ginsenosides against α-glycosidase and protein tyrosine phosphatase 1B (PTP1B). Results showed the anti-hyperglycaemic activities of the eight ginsenosides were in the order of 20(R)-dammarane-3β, 6α, 12β, 20, 25-pentol (25-OH-PPT) > 20(R)-25-methoxydammarane-3β, 12β, 20-tetrol (25-OCH3-PPT) > 20(R)-Protopanaxatriol (PPT) > 20(S)-panaxatriol (PT) > 20(R)-dammarane-3β, 12β, 20, 25-tetrol (25-OH-PPD) > 20(R)-25-methoxydammarane-3β, 12β, 20-triol (25-OCH3-PPD) > 20(R)-protopanaxadiol (PPD) > 20(S)-panaxadiol (PD), and 25-OH-PPT exerted stronger inhibitory activity than acarbose and Na3VO4. Meanwhile, simultaneous quantitative estimation results of eight ginsenosides suggested good linear regression within test ranges, precision, accuracy. 25-OH-PPT of the stems (leaves), flowers, and fruits contained 2.69, 3.31 and 7.20%, respectively.
CONCLUSIONS:
High performance liquid chromatography-evaporative light-scattering detector (HPLC-ELSD) method was proven to be simple, fast, and effective.