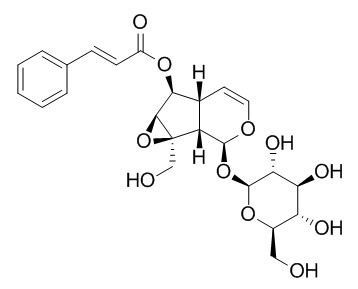
Picroside I
Picroside I is a hepatoprotective agent which is reported to be antimicrobial and used against hepatitis B. It has antioxidant, and anti-inflammatory activities, it may be the valuable anti-invasive drug candidates for cancer therapy by suppressing Collagenases and Gelatinases. Picroside I can enhance basic fibroblast growth factor(bFGF)-, staurosporine- or dbc-mitogen-activated protein (MAP)-induced neurite outgrowth from PC12D cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2022, 13:972825.
Antioxidants (Basel).2020, 9(6):466.
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Phytomedicine.2015, 22(11):1027-36
Eur J Pharmacol.2024, 978:176800.
Foods.2024, 13(11):1739.
Aging (Albany NY).2023, 15(24):15557-15577.
Food Chem.2024, 436:137768.
Chem Biol Interact.2016, 258:59-68
Molecules.2018, 23(9):E2121
Related and Featured Products
Life Sci. 2002 Aug 30;71(15):1821-35.
Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells.[Pubmed:
12151059]
METHODS AND RESULTS:
Picroside I and Picroside II caused a concentration-dependent (> 0.1 microM) enhancement of basic fibroblast growth factor (bFGF, 2 ng/ml)-, staurosporine (10 nM)- and dibutyryl cyclic AMP (dbcAMP, 0.3 mM)-induced neurite outgrowth from PC12D cells. Furthermore, picrosides-induced enhancements of the bFGF-action were markedly inhibited by GF109203X (0.1 microM), a protein kinase C inhibitor. The expression of phosphorylated MAP kinase was markedly increased by bFGF (2 ng/ml) and dbcAMP (0.3 mM), whereas that was not enhanced by staurosporine (10 nM). Picrosides had no effect on the phosphorylation of MAP kinase induced by bFGF or dbcAMP and also unaffected it in the presence of staurosporine. These results suggest that Picroside I and Picroside II enhance bFGF-, staurosporine- or dbcAMP-induced neurite outgrowth from PC12D cells, probably by amplifying a down-stream step of MAP kinase in the intracellular MAP kinase-dependent signaling pathway.
CONCLUSIONS:
Picroside I and Picroside II may become selective pharmacological tools for studying the MAP kinase-dependent signaling pathway in outgrowth of neurites induced by many kinds of neuritogenic substances including bFGF.
Pharmacognosy Res . 2017 Dec;9(Suppl 1):S53-S56.
Picroside I and Picroside II from Tissue Cultures of Picrorhiza kurroa[Pubmed:
29333043]
Abstract
Background: Picrorhiza kurroa (PK) belongs to Scrophulariaceae family and is a representative endemic, medicinal herb, widely distributed throughout the higher altitudes of alpine Himalayas from west to east, between 3000 and 4500 m above mean sea level.
Objective: The objective of the present study is to assess the production of Picroside I and Picroside II from tissue cultures of PK.
Materials and methods: Auxiliary shoot tips of PK were incubated in Murashige and Skoog medium supplemented with indole-3-butyric acid and kinetin phytohormones. The callus produced was collected at different time intervals and was processed for extraction of Picroside I and Picroside II followed by thin layer chromatography and high-performance liquid chromatography HPLC analysis.
Results: The maximum growth index was found to be 5.109 ± 0.159 at 16-week-old callus culture. The estimation of picroside-I and picroside-II was carried out by (HPLC) analysis; quantity of secondary metabolite found to be 16.37 ± 0.0007 mg/g for PK-I and 6.34 ± 0.0012 mg/g for PK-II.
Conclusion: This is the first attempt to produce the Picroside-I and II in large amount by the tissue culture technique. It can be observed that the method of callus culture can be used in production of secondary metabolites Picroside-I and II from PK.
Summary: Picrorhiza kurroa is a high value medicinal herb due to rich source of hepatoprotective metabolites, Picroside-I and Picroside-II. The medicinal importance of P. kurroa is due to its pharmacological properties like hepatoprotective, antioxidant (particularly in liver), antiallergic and antiasthamatic, anticancer activity particularly in liver and immunomodulatory. Shoot apices which were produced a good response was inoculated on selected medium i.e., on MS medium containing 2, 4 D (mg/l) + KN (1mg/l) for induction of callus. The initiation of callus was observed after 4weeks and it was light green and fragile Maximum growth was observed with 3% w/v of sucrose supplement. The callus culture was maintained and growth index was recorded after every subculture. The growth index was calculated from the obtained final dried weight divided by initial weight.Abbreviations Used: PK-Picrorhizakurroa, IBA-Indole-3-butyricacid, KN-Kinetin, 2,4D-2,4Dichlorophenoxy acetic acid.
Keywords: Picrorhiza kurroa; Picroside I; Picroside II; tissue cultures.
Phytother. Res., 1993, 7(6):402-7.
Antiinflammatory activity of the iridoids kutkin, picroside-1 and kutkoside from Picrorhiza kurrooa[Reference:
WebLink]
Powdered roots of Picrorhiza kurrooa (PK), its alcoholic extract (AEPK) and active constituents kutkin, Picroside I and kutkoside demonstrated antiinflammatory activity (AIA) in a variety of test models. Significant AIA was recorded in adjuvant-induced and formaldehyde arthritis in rats and mice. In carrageenan-induced oedema inhibitory activity was remarkably enhanced upon intraperitoneal treatment in rats and mice. Kutkin exhibited significant action in dextran-induced oedema in rats. It inhibited acetic acid induced vascular permeability in mice and leucocyte migration in rats. Kutkin lacked any analgesic, antipyretic or ulcerogenic effect.
Biochem Pharmacol. 1992 Jul 7;44(1):180-3.
Picroliv, picroside-I and kutkoside from Picrorhiza kurrooa are scavengers of superoxide anions.[Pubmed:
1321626]
Picroliv, the active principle of Picrorhiza kurrooa, and its main components which are a mixture of the iridoid glycosides, Picroside I and kutkoside, were studied in vitro as potential scavengers of oxygen free radicals.
METHODS AND RESULTS:
The superoxide (O2-) anions generated in a xanthine-xanthine oxidase system, as measured in terms of uric acid formed and the reduction of nitroblue tetrazolium were shown to be suppressed by picroliv, Picroside I and kutkoside. Picroliv as well as both glycosides inhibited the non-enzymic generation of O2- anions in a phenazine methosulphate NADH system. Malonaldehyde (MDA) generation in rat liver microsomes as stimulated by both the ascorbate-Fe2+ and NADPH-ADP-Fe2+ systems was shown to be inhibited by the Picroliv glycosides. Known antioxidants tocopherol (vitamin E) and butylated hydroxyanisole (BHA) were also compared with regard to their antioxidant actions in the above system. It was found that BHA afforded protection against ascorbate-Fe(2+)-induced MDA formation in microsomes but did not interfere with enzymic or non-enzymic O2- anion generation; and tocopherol inhibited lipid peroxidation in microsomes by both prooxidant systems and the generation of O2- anions in the non-enzymic system but did not interfere with xanthine oxidase activity.
CONCLUSIONS:
The present study shows that picroliv, Picroside I and kutkoside possess the properties of antioxidants which appear to be mediated through activity like that of superoxide dismutase, metal ion chelators and xanthine oxidase inhibitors.
Arab. J. Chem. 2011, 6(1):49-58.
Iridoid glycosides-Kutkin, Picroside I, and Kutkoside from Picrorrhiza kurroa Benth inhibits the invasion and migration of MCF-7 breast cancer cells through the down regulation of matrix metalloproteinases 1st Cancer Update[Reference:
WebLink]
Aim of the study Here, MCF-7 cell lines (Human breast cancer) were used to test whether P. kurroa extract (PE) and its isolated iridoid glycosides Picroside I (PS), Kutkoside (KS), and Kutkin (KT) exerts the anti-invasion activity via down-regulation of the expression of matrix metalloproteinases (MMPs). MMPs play an important role in solid tumor invasion and migration.
METHODS AND RESULTS:
The activity and expression of gelatinases (MMP-2 and MMP-9) and collagenases (MMP-1 and MMP-13), protein, and mRNA were detected by gelatin zymography, and RT-PCR. The migratory and invasive capacities of MCF-7 cell lines were measured by the wound scratch migration assay. The preliminary cytotoxicity testing was done by MTT assay and propidium iodide staining. Further the inhibition of inflammatory mediators was also done by quantification of nitrite inflammatory mediators.
The study showed that PE and its isolated iridoids glycosides PS, KS, and KT exhibited considerable cytotoxic potential in a dose-dependent manner. Further PE, PS, KS, and KT inhibited MCF-7 cell invasion and migration, and decreased MMP-2, 9 and MMP-1, 13 activities. Furthermore, PS, KS, and KT reduced MMPs expression at protein and mRNA levels, and suppression of the inflammatory mediators was also exhibited.
CONCLUSIONS:
Our results suggest that PS, KS, and KT may be the valuable anti-invasive drug candidates for cancer therapy by suppressing Collagenases and Gelatinases. PS, KS, and KT showed good results in comparison with PE. PS and KS exhibit almost comparable down regulation while KT exhibited maximum suppression of invasion, migration, and expression of MMPs.
Phytochem Anal. 2013 Nov-Dec;24(6):598-602.
A proposed biosynthetic pathway of picrosides linked through the detection of biochemical intermediates in the endangered medicinal herb Picrorhiza kurroa.[Pubmed:
23696248]
Picrorhiza kurroa Royle ex Benth is an important medicinal herb used in the preparation of several herbal drug formulations due to the presence of Picroside I (P-I) and picroside-II (P-II) along with other iridoid-glucosides derivatives.
The endangered status of P. kurroa coupled with lack of information on biosynthesis of Picroside Iand P-II necessitate deciphering the biosynthetic pathway for picrosides.
METHODS AND RESULTS:
LC with electrospray ionisation (ESI) and quadrupole time of flight combined with MS/MS was used to detect intermediates and assemble the picrosides biosynthetic pathway in P. kurroa. The presence of catalpol and aucubin, the major backbone structures of picrosides, along with intermediate metabolites boschnaloside, bartsioside and mussaenosidic acid, was confirmed in ESI negative mode with pseudomolecular ion peaks, that is, m/z 361, m/z 343, m/z 345, m/z 329 and m/z 375 ions and their fragmentation patterns.
CONCLUSIONS:
The picrosides biosynthetic pathway is expected to provide a reliable platform towards understanding the molecular components (genes/enzymes) of Picroside I and P-II biosynthesis in P. kurroa for their eventual utilisation in various applications.