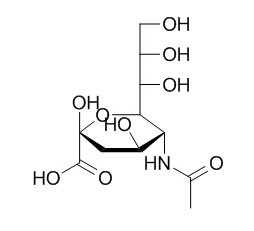
N-Acetylneuraminic acid
N-Acetylneuraminic acid is a nine-carbon, sialic acid monosaccharide commonly found in glycoproteins on cell membranes and in glycolipids such as gangliosides in mammalian cells.Synthesis of pyrrolidine analogues of N‐acetylneuraminic acid could as potential sialidase inhibitors. N-Acetylneuraminic acid-containing substances has growth-promoting effects on bifidobacteria, a number of N-acetylneuraminic acid-based compounds as potential rotavirus inhibitors.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Research2022, 6(6): 30-38.
iScience.2023, 26(9):107602.
Asian Journal of Chemistry2014, 26(22):7811-7816
Phytomedicine.2022, 110:154597.
Theoretical and Experimental Plant Physiology 2022, 34,53-62
Biochem Biophys Res Commun.2020, 522(1):40-46
University of East Anglia2023, 93969.
Molecules.2021, 26(2):E255.
Food Chem.2018, 262:78-85
J Ethnopharmacol.2019, 244:112074
Related and Featured Products
Infect Immun. 2014 Dec;82(12):5235-45.
Aeromonas salmonicida binds differentially to mucins isolated from skin and intestinal regions of Atlantic salmon in an N-acetylneuraminic acid-dependent manner.[Pubmed:
25287918]
Aeromonas salmonicida subsp. salmonicida infection, also known as furunculosis disease, is associated with high morbidity and mortality in salmonid aquaculture. The first line of defense the pathogen encounters is the mucus layer, which is predominantly comprised of secreted mucins.
METHODS AND RESULTS:
Here we isolated and characterized mucins from the skin and intestinal tract of healthy Atlantic salmon and studied how A. salmonicida bound to them. The mucins from the skin, pyloric ceca, and proximal and distal intestine mainly consisted of mucins soluble in chaotropic agents. The mucin density and mucin glycan chain length from the skin were lower than were seen with mucin from the intestinal tract. A. salmonicida bound to the mucins isolated from the intestinal tract to a greater extent than to the skin mucins. The mucins from the intestinal regions had higher levels of sialylation than the skin mucins. Desialylating intestinal mucins decreased A. salmonicida binding, whereas desialylation of skin mucins resulted in complete loss of binding. In line with this, A. salmonicida also bound better to mammalian mucins with high levels of sialylation, and N-Acetylneuraminic acid appeared to be the sialic acid whose presence was imperative for binding.
CONCLUSIONS:
Thus, sialylated structures are important for A. salmonicida binding, suggesting a pivotal role for sialylation in mucosal defense. The marked differences in sialylation as well as A. salmonicida binding between the skin and intestinal tract suggest interorgan differences in the host-pathogen interaction and in the mucin defense against A. salmonicida.
J Med Chem. 1996 Mar 15;39(6):1314-20.
Synthesis and biological evaluation of N-acetylneuraminic acid-based rotavirus inhibitors.[Pubmed:
8632438]
Rotavirus can cause severe gastrointestinal disease, especially in infants and young children, and is particularly prevalent in Third-World countries. Therefore, the development of potential inhibitors of this virus is of great interest.
METHODS AND RESULTS:
The present study describes the synthesis and in vitro biological evaluation of a number of N-Acetylneuraminic acid-based compounds as potential rotavirus inhibitors.
CONCLUSIONS:
Our data suggests that it is indeed possible to inhibit adhesion of the virus, and hence in vitro replication, with carbohydrate-based molecules, although this inhibition does appear to be strain dependent.
Biosci. Biotech. Biochem. 1994, 58(9):1720-2.
Growth-promoting Effects of N-Acetylneuraminic Acid-containing Substances on Bifidobacteria.[Reference:
WebLink]
METHODS AND RESULTS:
The use of N-Acetylneuraminic acid, sialyl-lactose, and glyco-macropeptide by bifidobacteria and lactobacilli, and their growth-promoting effects on B. longum, B. breve, B. bifidum, and B. infantis were investigated.
CONCLUSIONS:
The data presented here suggest that fortification with N-Acetylneuraminic acid-containing substances of infant formula may provide formula-fed infants with a function that human milk possesses.
Carbohydr Res. 2015 Jan 30;402:133-45.
Characterization of the N-acetylneuraminic acid synthase (NeuB) from the psychrophilic fish pathogen Moritella viscosa.[Pubmed:
25498013]
Moritella viscosa is a Gram-negative psychrophilic bacterium that causes winter ulcer disease in Atlantic salmon and cod. Its genome reveals that it possesses the ability to synthesize sialic acids. Indeed, sialic acid can be isolated from the bacterium and when analyzed using HPLC-MS/MS, the presence of N-Acetylneuraminic acid was confirmed. Thus, the N-Acetylneuraminic acid synthase NeuB from M. viscosa (MvNeuB) was recombinantly produced and characterized.
METHODS AND RESULTS:
The optimum pH and temperature for MvNeuB activity are 7.5 and 30 °C, respectively. The KM for N-acetylmannosamine and phosphoenolpyruvate is 18±5 and 0.8±0.2 mM, respectively. The kcat value (∼225 min(-1)) for both N-acetylmannosamine and phosphoenolpyruvate is the highest turnover number found for an enzyme in this class until the date. A calorimetric study of MvNeuB shows that the enzyme has a two-step transition peak probably reflecting the two domains these proteins consist of. MvNeuB is less stable at higher temperature and has a high catalytic activity at lower temperature compared to mesophilic counterparts. Enzymes from psychrophilic organisms are generally cold adapted meaning they can maintain adequate function near the freezing point of water. Cold adapted enzymes are catalytically more efficient at lower temperature and are more thermo-labile compared to their mesophilic counterparts.
CONCLUSIONS:
MvNeuB is a typical cold adapted enzyme and could be further explored for production of sialic acids and derivates at low temperatures.