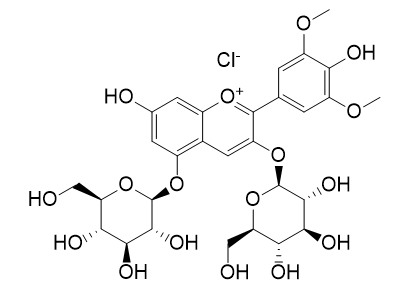
Malvidin-3,5-O-diglucoside chloride
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Med Food.2021, 24(3):209-217.
Molecules.2018, 23(9):E2121
Phytomedicine.2016, 23(4):331-9
Arch Toxicol.2017, 91(10):3225-3245
J Chromatogr A.2024, 1714:464544.
ACS Synth Biol.2022, doi: 10.1021.
Dis Markers.2022, 2022:2380879.
J Food Compos Anal2017, 62:197-204
Mutlu Yanic S, Ates EG. JOTCSA.2023, 10(4);893-902.
PLoS One.2017, 12(8):e0181191
Related and Featured Products
Electroanalysis, 2010, 19(17):1779-1786.
Redox Behavior of Anthocyanins Present in Vitis vinifera L.[Reference:
WebLink]
Voltammetric techniques were employed to study the electrochemical behavior of several anthocyanins.
METHODS AND RESULTS:
The redox behavior of anthocyanins with the same basic structure, the influence of glycosylation on the redox behavior of anthocyanins derived from different anthocyanidins, and the influence of methoxylation were investigated. The anthocyanins used in this study were malvidin-3-O-glucoside chloride, malvidin-3,5-di-O-glucoside chloride(Malvidin-3,5-O-diglucoside chloride), cyanidin-3-O-glucoside chloride, cyanidin-3,5-di-O-glucoside chloride, peonidin-3-O-glucoside chloride, delphinidin-3-O-glucoside chloride and the anthocyanidin petunidin chloride, all of them present in Vitis vinifera L.
CONCLUSIONS:
All hydroxyl groups of the anthocyanins can be electrochemically oxidized and the anthocyanins studied revealed a complex and pH dependent oxidation process, with the occurrence of adsorption and of oxidation products blocking the electrode surface.