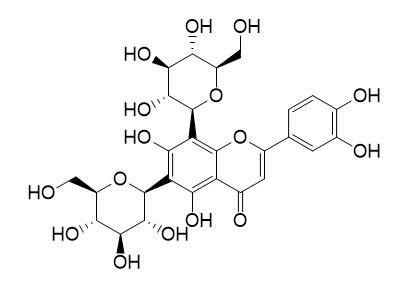
Lucenin II
Lucenin II has anticonvulsant, antibacterial, anti-inflammatory and antihepatotoxic activity.
Lucenin II caused a decrease in the percentage of spore germination, protonemal development and root growth.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
ACS Omega.2023, 8(36):32424-32431.
J. Soc. Cosmet. Sci. Korea2016, 163-171
J of Essential Oil Research2019, 1677272
The Japan Society for Analy. Chem.2017, 66(8):613-617
Curr Top Med Chem.2020, 20(21):1898-1909.
J Pharm Pharmacol.2023, 75(9):1225-1236.
Adv. Anim. Vet. Sci.2024, 12(4):732-741
Int J Med Sci.2024, 21(15):2883-2896
Nat Prod Sci.2019, 25(3):238
ACS Synth Biol.2022, 11(10):3296-3304.
Related and Featured Products
Iran J Pharm Res . Winter 2017;16(Suppl):46-57.
Bioactivity-guided Study of Passiflora caerulea L. Leaf Extracts[Pubmed:
29844775]
Passiflora species were known by their anticonvulsant, anxiolytic and sedative activities. The aim of this study was to investigate the chemical composition of the most active leaf extract of Passiflora caerulea L. grown in Egypt. The ethanolic extract of the leaves exhibited higher activity than aqueous extract as anticonvulsant (63% potency relative to carbamazepine), analgesic (70% potency relative to indomethacin), antioxidant (71% potency relative to vitamin E), anti-inflammatory (90% potency relative to indomethacin) and antipyretic (90% potency relative to paracetamol). Fractions obtained successively from the ethanolic extract were then subjected to the same biological testing demonstrating that the ethyl acetate fraction was the most active in all activities (50, 96, 80, 63 % potency relative to reference standards used in each of the selected activity, respectively) followed by n-butanol then n-hexane and chloroform fractions. Purification of the anticonvulsant sub-fractions obtained by column chromatography of ethyl acetate fraction, led to the isolation of three compounds that were identified by physical and spectroscopic techniques as Lucenin II (1), 4-hydroxycinnamic acid (2) and Chrysin 6-C-β-D-glucoside (3). The amount of Chrysin 6-C-β-D-glucoside was found to be 0.0184 g % w/w of the dried leaves using HPLC method that showed linearity (R2 = 0.9996) over the range 0.015-0.25 mg/mL. C-glycosyl flavones and hydroxycinnamic acid derivatives may thus be the responsible principles for the biological activity of the plant under investigation. Moreover, RAPD technique was performed for the genetic characterization and authentication of the plant, where 106 fragments were recorded after DNA amplification with fifteen primers.
Phytochemistry . 2003 Apr;62(7):1145-51
Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW (Bryophyta) and Raphanus sativus L (Magnoliophyta)[Pubmed:
12591270]
Dried mosses (five moss species) were progressively extracted and subjected to a four-step Craig distribution. Seven pure flavonoids were isolated and identified. The flavonoids were the flavones apigenin, apigenin-7-O-triglycoside, lucenin-2, luteolin-7-O-neohesperidoside, saponarine and vitexin; and the biflavonoid bartramiaflavone and they were submitted to biological tests. The tests were performed in vitro on spore germination and protonemal growth of the moss Tortula muralis and on seed germination and root growth of Raphanus sativus. Flavonoids caused a decrease in the percentage of spore germination, protonemal development and root growth. In addition they caused morphological alterations, such as forked tips, swollen apices, rounded cells and early formation of brood cells in the protonemata. Data were discussed in relation to the presence of allelochemicals in mosses.
Pharm Biol . 2011 Aug;49(8):840-9.
Secondary metabolites of Centaurea calolepis and evaluation of cnicin for anti-inflammatory, antioxidant, and cytotoxic activities[Pubmed:
21612369]
Context: Centaurea L. (Astreaceae) species are used as herbal remedies in Turkey. Centaurea calolepis Boiss. is an endemic species of Anatolia that has not been subjected to phytochemical studies except essential oil analysis.
Objective: Secondary metabolite determination, isolation and structure elucidation of pure compounds were performed on C. calolepis. Cnicin, which is the main component of several Centaurea species, was tested for its in vitro anti-inflammatory, antioxidant and cytotoxic activities.
Materials and methods: Chloroform and methanol extracts of the aerial parts of C. calolepis were subjected to isolation process using column chromatography. The structures of the compounds were characterized by 1D- and 2D-NMR experiments. Thin-layer chromatography and high performance liquid chromatography were used in determination of phenolics. Cnicin was subjected to a panel of cellular assays to test for inhibition of nuclear factor κB (NF-κB), inducible nitric oxide synthase (iNOS), reactive oxygen species and cytotoxicity.
Results: Cnicin, lucenin-2, schaftoside and 3-O-feruloylquinic acid were isolated from C. calolepis extracts. Vicenin-2, vitexin, isovitexin, homoorientin, rutin, orientin, luteolin-7-O-glycoside and chlorogenic acid were determined in fractions. Cnicin showed inhibition of NF-κB and inhibition of iNOS activity with IC₅₀ Values of 1.8 and 6.5 μM, respectively. Cytotoxic activity of cnicin was observed toward pig kidney epithelial (LLC-PK₁₁), human malignant melanoma (SK-MEL) and human ductal carcinoma (BT-549) cells with IC₅₀ values of 23.3, 14.0 and 18.3 μM, respectively.
Discussion and conclusion: This is the first detailed report of secondary metabolites of C. calolepis. Evaluation of biological activity of cnicin establishes the potential of this compound as an anti-inflammatory and cytotoxic agent.
Phytochemistry . 1999 Dec;52(8):1479-82
Antibacterial activity of pure flavonoids isolated from mosses[Pubmed:
10647220]
Seven pure flavonoids were isolated and identified from five moss species. The flavonoids were the flavones apigenin, apigenin-7-O-triglycoside, lucenin-2, luteolin-7-O-neohesperidoside, saponarine and vitexin; and the biflavonoid bartramiaflavone. Some of these flavonoids were shown to have pronounced antibacterial effects against Enterobacter cloaceae, E. aerogenes and Pseudomonas aeruginosa (minimal bacteriostatic concentration MIC in the range of 4-2048 micrograms/ml). Because of their antibacterial spectrum mainly active against Gram negative bacterial strains, responsible for severe opportunistic infections and resistant to common antibacterial therapy, these flavonoids may be important tools in antibacterial strategies.
Food Chem . 2014 Apr 15;149:244-252.
First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties[Pubmed:
24295703]
RP-LC-DAD-ESI-MS-MS separation/identification protocol has been employed for the identification and characterisation of nine C- and O-glycosyl flavonoids in Moro (Citrus sinensis (L.) Osbeck) juice grown in Southern Italy. For the first time we reported the presence of five C-glycosyl flavones (lucenin-2, vicenin-2, stellarin-2, lucenin-2 4'-methyl ether and scoparin), a 3-hydroxy-3-methylglutaryl glycosyl flavonol (3-hydroxy-3-methylglutaryl glycosyl quercetin) and a flavone O-glycosides (chrysoeriol 7-O-neoesperidoside). Moreover, the influence of the identified C- and O-glycosyl flavonoids on the total antioxidant activity of crude juice has been evaluated on the basis of its ability to scavenge DPPH•, OH• and ABTS•+ radicals and to reduce iron.
Biomol Ther (Seoul) . 2016 Nov 1;24(6):630-637.
Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica[Pubmed:
27302962]
The chemical components and biological activity of Camellia mistletoe, Korthalsella japonica (Loranthaceae) are relatively unknown compared to other mistletoe species. Therefore, we investigated the phytochemical properties and biological activity of this parasitic plant to provide essential preliminary scientific evidence to support and encourage its further pharmaceutical research and development. The major plant components were chromatographically isolated using high-performance liquid chromatography and their structures were elucidated using tandem mass spectrometry and nuclear magnetic resonance anlysis. Furthermore, the anti-inflammatory activity of the 70% ethanol extract of K. japonica (KJ) and its isolated components was evaluated using a nitric oxide (NO) assay and western blot analysis for inducible NO synthase (iNOS) and cyclooxygenase (COX)-2. Three flavone di-C-glycosides, lucenin-2, vicenin-2, and stellarin-2 were identified as major components of KJ, for the first time. KJ significantly inhibited NO production and reduced iNOS and COX-2 expression in lipopolysaccharide-stimulated RAW 264.7 cells at 100 μg/ mL while similar activity were observed with isolated flavone C-glycosides. In conclusion, KJ has a simple secondary metabolite profiles including flavone di-C-glycosides as major components and has a strong potential for further research and development as a source of therapeutic anti-inflammatory agents.
Planta Med . 1992 Dec;58(6):544-548.
Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis[Pubmed:
1484895]
From the leaves of Allophyllus edulis var. edulis and Allophyllus edulis var. gracilis nine C-glycosylflavones have been isolated and identified as schaftoside (8), vicenin-2 (9), lucenin-2 (10), isovitexin 2"-O-rhamnoside (11), cerarvensin 2"-O-rhamnoside (12), vitexin 2"-O-rhamnoside (13), isoorientin 2"-O-rhamnoside (15), orientin 2"-O-rhamnoside (16) and saponarin (17). In addition, gallic acid (2), the phenol C-glycosides bergenin (3) and 11-O-galloylbergenin (4), three flavonol 3-O-rhamnosides and a new C-glycosylflavone identified as mollupentin 2"-O-rhamnoside (14) were obtained from the leaves of Allophyllus edulis var. edulis. Their structures were elucidated on the basis of chemical and spectral data. For the first time the C-glycosylflavones were found to have remarkable anti-hepatotoxic activities against CCl4 and galactosamine cytotoxicity in primary cultured rat hepatocytes. Structure-activity relationships are discussed.
Asian Pac J Trop Biomed . 2011 Oct;1(5):376-80.
Analytical characterization and structure elucidation of metabolites from Aspergillus ochraceus MP2 fungi[Pubmed:
23569796]
Objective: To isolate and characterize the bioactive secondary metabolites from Aspergillus ochraceus (A. ochraceus) MP2 fungi.
Methods: The anti bacterial activity of marine sponge derived fungi A. ochraceus MP2 was thoroughly investigated against antagonistic human pathogens. The optimum inhibitory concentration of the fungi in the elite solvent was also determined. The promising extracts that showed good antimicrobial activity were subjected to further analytical separation to get individual distinct metabolites and the eluants were further identified by GC MS instrumental analysis. The molecular characterization of the elite fungal strains were done by isolating their genomic DNA and amplify the internal transcribed spacer (ITS) region of 5.8s rRNA using specific ITS primer. The novelty of the strain was proved by homology search tools and elite sequences was submitted to GENBANK.
Results: Three bioactive compounds were characterized to reveal their identity, chemical formula and structure. The first elutant was identified asα- Campholene aldehyde with chemical formula C10 H16 O and molecular weight 152 Da. The second elutant was identified as Lucenin-2 and chemical formula C27 H30 O16 and molecular weight 610 Da. The third elutant was identified as 6-Ethyloct- 3-yl- 2- ethylhexyl ester with Chemical formula C26 H42 O4 with molecular weight 418 Da.
Conclusions: The isolated compounds showed significant antimicrobial activity against potential human pathogens. Microbial secondary metabolites represent a large source of compounds endowed with ingenious structures and potent biological activities.