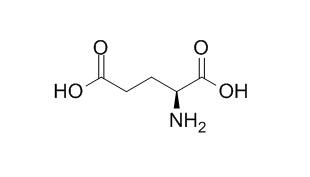
L-Glutamic acid
L-Glutamic acid is probably an excitatory transmitter of major significance in the mammalian central nervous system. L-Glutamic acid and L-lysine as useful building blocks for the preparation of bifunctional DTPA-like ligands.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Progress In Microbes & Molecular Biology2025, 8,1:a0000470.
Psychopharmacology (Berl).2020, 10.1007
Molecules.2015, 20(10):19172-88
Asian J Beauty Cosmetol2016, 14(3):249-257
Food and Fermentation Industries2018, 44(371)
Natural Product Sciences2024, 30(1):8-13.
Front Chem.2024, 12:1385844.
Industrial Crops and Products2022, 186:115298
BMC Complement Med Ther.2023, 23(1):264.
Int J Mol Sci.2022, 23(20):12516.
Related and Featured Products
Nature, 1974 , 248 (5451) :804-5.
Spinal interneurone excitation by conformationally restricted analogues of L-glutamic acid[Reference:
WebLink]
L-Glutamic acid is probably an excitatory transmitter of major significance in the mammalian central nervous system1.
METHODS AND RESULTS:
The L-Glutamic acid molecule is relatively flexible, and in an attempt to gain some insight into its possible shape(s) during activation of receptors associated with excitation of central neurones, a study was made of four conformationally restricted analogues: (±)-cis-1-aminocyclohexane-1,3-dicarboxylic acid (‘cyclo-glutamic’ acid), ibotenic acid, kainic acid and its dihydro derivative.
Bioconjug. Chem., 1999 , 10 (1) :137-40.
L-Glutamic acid and L-lysine as useful building blocks for the preparation of bifunctional DTPA-like ligands.[Reference:
WebLink]
METHODS AND RESULTS:
Bisalkylation of suitably protected L-Glutamic acid and L-lysine derivatives with tert-butyl N-(2-bromoethyl)iminodiacetate 2, followed by deprotection of the omega functional group affords N, N-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-Glutamic acid 1-(1, 1-dimethylethyl) ester 4 and N2,N2-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-lysine 1,1-dimethylethyl ester 7. Such compounds feature a carboxylic or an amino group, respectively, which are available for conjugation with a suitable partner via formation of an amide bond. The conjugates, which can be prepared in this way, contain a chelating subunit in which all five acetic residues of DTPA are available for the complexation of metal ions. Direct bisalkylation of glycine with 2 promptly gives N, N-bis[2-[bis[2-(1,1-dimethylethoxy)-2-oxoethyl]amino]ethyl]glycine 11.
CONCLUSIONS:
The latter allows to achieve conjugates in which the central acetic group of DTPA is selectively converted into an acetamide.