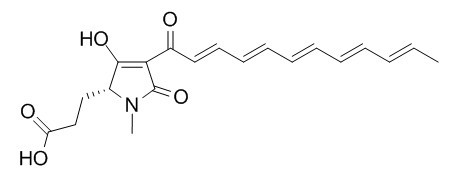
Fuligorubin A
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochemical Systematics and Ecology2018, 81
Korean Herb. Med. Inf.2021, 9(2):231-239.
Molecules.2020, 25(7):1625.
Cell Physiol Biochem.2017, 44(4):1381-1395
NanoBioScience2024, v13:3:115.
J Microbiol Biotechnol.2024, 35:e2408022.
Research Square2020, doi: 10.21203.
Toxicol In Vitro.2023, 86:105521.
Food Chem.2021, 360:130063.
Neurochem Int.2020, 133:104629
Related and Featured Products
Tetrahedron,1992,48(6): 1145–1174.
Further reactions of t-butyl 3-oxobutanthioate and t-butyl 4-diethyl-phosphono-3-oxobutanthioate : Carbonyl coupling reactions, amination, use in the preparation of 3-acyltetramic acids and application to the total synthesis of fuligorubin A.[Reference:
WebLink]
METHODS AND RESULTS:
The use of t-butyl-3-oxobutanthioate (1) and t-butyl 4-diethylphosphono-3-oxobutanthioate (2) for the preparation of homologated derivatives suitable for amination in the presence of silver (I) trifluoroacetate to afford the corresponding β-ketoamides is discussed. In particular Wadsworth-Emmons coupling reactions of (2) with various carbonyl compounds gave good yields of E-substituted products.
CONCLUSIONS:
Many of the β-ketoamides were shown to be suitable precursors for 3-acyltetramic acids using a Dieckmann cyclisation with tetra-n-butyl ammonium fluoride as the cyclising base. These new reactions were applied to the total synthesis of the polyene 3-acyltetramic acid Fuligorubin A.
Tetrahedron Letters,1988,29(45):5829–5832.
Use of t-butyl 4-diethylphosphono-3-oxobutanethioate for tetramic acid synthesis: Total synthesis of the plasmodial pigment fuligorubin A[Reference:
WebLink]
METHODS AND RESULTS:
A short, efficient synthesis of the yellow slime mould pigment Fuligorubin A (1) has been achieved using coupling of t-butyl 4-diethylphosphono-3-oxobutanethioate with deca-2,4,6,8-tetraenal and subsequent substitution with a glutamic acid derivative followed by Dieckmann cyclisation.