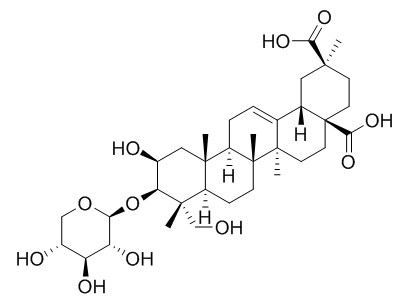
Esculentoside E
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Free Radic Biol Med.2021, 166:104-115.
Journal of Apicultural Research2021, 60(1).
Immunopharmacol Immunotoxicol.2024, 46(4):496-508.
Food Chem X.2024, 21:101127.
Int J Mol Med.2016, 37(2):501-8
Phytomedicine.2022, 99:154025.
Heliyon.2024, 10(7):e28364.
Oncol Rep.2021, 46(2):166.
Appl. Sci. 2021, 11(22),10569
Appl. Sci. 2024, 14(13), 5815
Related and Featured Products
Acta pharmaceutica sinica, 1984.
STUDIES ON THE ACTIVE PRINCIPLES OF THE CHINESE DRUG "SHANG LU" (PHYTOLACCA ESCULENTA VAN HO UTTE)Ⅱ. THE ISOLATION AND STRUCTURE OF ESCULENTOSIDE E AND F.[Reference:
WebLink]
In a previous paper we reported the isolation of esculentoside A, B, C and D from Phytolacca eseulenta Van Houtte.In pharmacological experiments the total saponins and esculentoside A exerted considerable enhancement of phagocytic function of leucocytes and promoted DNA synthesis in mice. .
METHODS AND RESULTS:
In further search for active principles, four kinds of crystals (Ⅰ), (Ⅱ), (Ⅲ) and (Ⅳ) were isolated from the 0.01 M pH 7.3 phosphate buffer extract of the plant by silica gel column chromatography. On the basis of chemical properties and spectral data (NMR, ~(13)CNMR, UV, IR, Ms spectra), crystal (Ⅰ) was shown to be 2-hydroxyl esculentic acid, i.e. jaligonic acid which has been considered to have good antiinflammatory action; crystal (Ⅱ) was found to be 3-O-β-D-xylopyranosyl-2-hydroxylesculentic acid and was named as esculentosid E which is identical to phytolaccoside G from Phytolacca americana. Crystal (Ⅲ) is 3-O(β-D-glucopyranosyl-β-D-xylopyranosyl)_(1→4)-2-hydroxyl esculentic acid named as esculentoside F.
CONCLUSIONS:
Esculentoside E was first obtained from this plant and esculentoside F so far has not been reported in literature. Because of the scanty sample of crystal (Ⅳ), its structure is still under investigation.