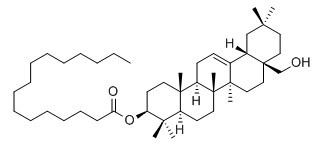
Erythrodiol 3-palmitate
Erythrodiol 3-palmitate has antitumor activity, it inhibited the proliferation of K562 cells with the inhibition rate of 47% at 100 ug/mL.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Trop J Nat Prod Res2023, 7(12):5611-5615.
Molecules2022, 27(9):2827.
Korean J. Food Sci. & Technol.2022, 54(2):241-246
Comparative Clinical Pathology 2021, 30:961-971.
Molecules.2022, 27(7):2360.
Elife.2021, 10:e68058.
Br J Pharmacol.2016, 173(2):396-410
J Colloid Interface Sci.2022, 622:298-308.
Chung Shan Medical University2020, US20200323790A1
Food Hydrocolloids2024, 57:110432
Related and Featured Products
Chinese Journal of Medicinal Chemistry,2009,19(3):195-9.
Triterpenoidal constituents of Pericampylus glaucus and their antitumor activity in vitro[Reference:
WebLink]
To investigate triterpenoidal constituents of Pericampylus glaucus(Lam.)Merr.and to eva-luate their antitumor activities.
METHODS AND RESULTS:
Various chromatographic means were used for the isolation of triterpenes and the compounds obtained were identified by the spectroscopic method.Antitumor activities were also assayed by MTT method. Five triterpenes were isolated from Pericampylus glaucus and identified as hopenone-B(1),hopenol-B(2),22-hydroxyhopan-3-one(3),Erythrodiol 3-palmitate(4),and 5β,24-cyclofriedelan-3-one(5),respectively.Among them,compound 4 inhibited the proliferation of K562 cells with the inhibition rate of 47% at 100 μg·mL-1,while others did not show inhibitory effect at the concentration tested.
CONCLUSIONS:
Compounds 1-5 were isolated from the genus Pericampylus for the first time.Compound 4 is the first reported antitumor constituent of Pericampylus glaucus and its antitumor activity was also assayed for the first time.The 13C-NMR data for 2 and the 1H-NMR and 13C-NMR data for 1 and 5 were reported for the first time.
Planta Med 1975; 27(1): 89-92.
ISOLATION OF ERYTHRODIOL 3–PALMITATE FROM BUMELIA OBTUSIFOLIA.[Reference:
WebLink]
METHODS AND RESULTS:
Chromatographie separation of the methanol extract of the aerial portion of Bumelia obtusifolia R. & S. yielded lupeol acetate, β-amyrine acetate, β–amyrine, taraxerol, and Erythrodiol 3-palmitate.