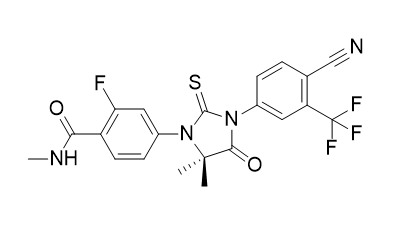
Enzalutamide (MDV3100)
Enzalutamide (MDV3100) is an androgen-receptor (AR) antagonist with IC50 of 36 nM in LNCaP cells. Enzalutamide is shown to increase autophagy.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plants (Basel).2024, 13(19):2793.
Int J Biol Macromol.2021, 199:189-200.
Evid Based Complement Alternat Med.2021, 8855980.
Phytother Res.2015, 29(7):1088-96
Molecules.2021, 26(8):2161.
Cancer Manag Res.2019, 11:483-500
Pharmacia2024, 71:1-9.
Int J Oncol.2019, 55(1):320-330
Mol Med Rep.2023 Oct;28(4):193.
Process Biochemistry2019, 87:213-220
Related and Featured Products
Lancet, 2010 Apr 24;375(9724):1437-46.
Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study.[Pubmed:
20398925]
MDV3100 is an androgen-receptor antagonist that blocks androgens from binding to the androgen receptor and prevents nuclear translocation and co-activator recruitment of the ligand-receptor complex. It also induces tumour cell apoptosis, and has no agonist activity. Because growth of castration-resistant prostate cancer is dependent on continued androgen-receptor signalling, we assessed the antitumour activity and safety of MDV3100 in men with this disease.
METHODS AND RESULTS:
This phase 1-2 study was undertaken in five US centres in 140 patients. Patients with progressive, metastatic, castration-resistant prostate cancer were enrolled in dose-escalation cohorts of three to six patients and given an oral daily starting dose of MDV3100 30 mg. The final daily doses studied were 30 mg (n=3), 60 mg (27), 150 mg (28), 240 mg (29), 360 mg (28), 480 mg (22), and 600 mg (3). The primary objective was to identify the safety and tolerability profile of MDV3100 and to establish the maximum tolerated dose. The trial is registered with ClinicalTrials.gov, number NCT00510718.We noted antitumour effects at all doses, including decreases in serum prostate-specific antigen of 50% or more in 78 (56%) patients, responses in soft tissue in 13 (22%) of 59 patients, stabilised bone disease in 61 (56%) of 109 patients, and conversion from unfavourable to favourable circulating tumour cell counts in 25 (49%) of the 51 patients. PET imaging of 22 patients to assess androgen-receptor blockade showed decreased (18)F-fluoro-5alpha-dihydrotestosterone binding at doses from 60 mg to 480 mg per day (range 20-100%). The median time to progression was 47 weeks (95% CI 34-not reached) for radiological progression. The maximum tolerated dose for sustained treatment (>28 days) was 240 mg. The most common grade 3-4 adverse event was dose-dependent fatigue (16 [11%] patients), which generally resolved after dose reduction.
CONCLUSIONS:
We recorded encouraging antitumour activity with MDV3100 in patients with castration-resistant prostate cancer. The results of this phase 1-2 trial validate in man preclinical studies implicating sustained androgen-receptor signalling as a driver in this disease.
US2007254933 A1
Original document: US2007254933 (A1) ― 2007-11-01[Reference:
WebLink]
Enzalutamide is evaluated by an artificial AR response reporter system in a hormone refractory prostate cancer cell line. In this system, the prostate cancer LNCaP cells are engineered to stably express about 5-fold higher level of AR than endogenous level. The exogenous AR has similar properties to endogenous AR in that both are stabilized by a synthetic androgen R1881. The AR-over expressed cells are also engineered to stably incorporate an AR response reporter and the reporter activity of these cells shows features of hormone refractory prostate cancer. The antagonistic activity of Enzalutamide is tested in the presence of 100 pM of R1881. Engineered LNCaP cells are maintained in Iscove's medium containing 10% fetal bovine serum (FBS). Two days prior to Enzalutamide treatment, the cells are grown in Iscove's medium containing 10% charcoal-stripped FBS (CS-FBS) to deprive of androgens. The cells are split and grown in Iscove's medium containing 10% CS-FBS with 100 pM of R1881 and increasing concentrations of Enzalutamide. After two days of incubation, reporter activities are assayed.
Science,2009 May 8;324(5928):787-90.
Development of a second-generation antiandrogen for treatment of advanced prostate cancer.[Pubmed:
19359544]
Cell lines:LNCaP or LNCaP/AR cells
Concentrations: 0-10 μM
Incubation Time: 1-4 days
Method:
Enzalutamide is diluted in DMSO. LNCaP or LNCaP/AR cells (104 cells/well) are androgen-starved by growth in media containing 5-10% charcoal-stripped serum for 3-5 days. Then the cells are challenged with various concentrations of Enzalutamide in media containing 5-10% charcoal-stripped serum.
Science,2009 May 8;324(5928):787-90.
Development of a second-generation antiandrogen for treatment of advanced prostate cancer.[Pubmed:
19359544]
Animal Models: Castration-resistant LNCaP/HR xenografts in male SCID mice
Dosages:10 mg/kg
Administration: Administered via gavage daily