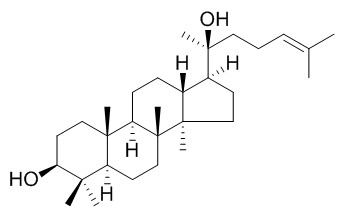
Dammarenediol II
Dammarenediol II may have the ability to prevent diabetic microvascular complications, including diabetic retinopathy, it can inhibit vascular endothelial growth factor (VEGF)-induced intracellular reactive oxygen species generation and stress fiber formation and vascular endothelial-cadherin disruption. The medicinally important dammarenediol II can be ectopically produced in tobacco, and the production of dammarenediol-II in tobacco plants allows them to adopt a viral defense system.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Expert Opin Ther Targets.2024, :1-11.
Molecules.2022, 27(2):451.
J Nutr Biochem.2022, 107:109064.
Molecules.2024, 29(24):5983.
J. Soc. Cosmet. Sci. Korea2016, 163-171
J.Food Pharm.Sci.2024, 12(2), 116-124.
J of the Korean Society of Food Science and Nutrition2016, 45(7):1017-1025
Trop J Nat Prod Res2023, 7(12):5611-5615.
Chemistry of Plant Raw Materials2019, 4:135-147
Int J Mol Sci.2023, 24(18):14077.
Related and Featured Products
Plant Cell Physiol. 2012 Jan;53(1):173-82.
Dammarenediol-II production confers TMV tolerance in transgenic tobacco expressing Panax ginseng dammarenediol-II synthase.[Pubmed:
22102695]
Panax ginseng is one of the famous medicinal plants. Ginsenosides, a class of tetracyclic triterpene saponins, are mainly responsible for its pharmacological activity. Most ginsenosides are composed of Dammarenediol II aglycone with various sugar moieties. Dammarenediol II synthase is the first enzyme in the biosynthesis of ginsenosides.
METHODS AND RESULTS:
Here, we report that transgenic tobacco expressing the P. ginseng Dammarenediol II synthase gene (PgDDS) produced Dammarenediol II, and conferred resistance to Tobacco mosaic virus (TMV). Upon infection with TMV, lesions developed more rapidly in transgenic tobacco plants, and their size was smaller than those of wild-type plants. Transgenic tobacco plants showed a low level of both the viral titer and mRNA accumulation of TMV coat protein (CP) compared with the wild type. The production of Dammarenediol II in transgenic tobacco stimulated the expression of tobacco pathogenesis-related genes (PR1 and PR2) under both virus-untreated and -treated conditions. When the leaves of wild-type plants were inoculated with a mixture of TMV and Dammarenediol II, the leaves exhibited a reduced viral concentration and TMV-CP expression than those receiving TMV treatment alone. When the leaves of P. ginseng were infected with TMV, transcription of PgDDS was significantly increased. Transgenic P. ginseng plants harboring a β-glucuronidase (GUS) gene driven by the PgDDS promoter were constructed. The GUS expression was activated when the transgenic ginseng plants were treated with TMV.
CONCLUSIONS:
These results indicate that the medicinally important Dammarenediol II can be ectopically produced in tobacco, and the production of Dammarenediol II in tobacco plants allows them to adopt a viral defense system.
Phytother Res. 2015 Dec;29(12):1910-6.
Dammarenediol-II Prevents VEGF-Mediated Microvascular Permeability in Diabetic Mice.[Pubmed:
26400610]
Diabetic retinopathy is a major diabetic complication predominantly caused by vascular endothelial growth factor (VEGF)-induced vascular permeability in the retina; however, treatments targeting glycemic control have not been successful.
METHODS AND RESULTS:
Here, we investigated the protective effect of Dammarenediol II, a precursor of triterpenoid saponin biosynthesis, on VEGF-induced vascular leakage using human umbilical vein endothelial cells (HUVECs) and diabetic mice. We overproduced the compound in transgenic tobacco expressing Panax ginseng Dammarenediol II synthase gene and purified using column chromatography. Analysis of the purified compound using a gas chromatography-mass spectrometry system revealed identical retention time and fragmentation pattern to those of authentic standard Dammarenediol II. Dammarenediol II inhibited VEGF-induced intracellular reactive oxygen species generation, but it had no effect on the levels of intracellular Ca(2+) in HUVECs. We also found that Dammarenediol II inhibited VEGF-induced stress fiber formation and vascular endothelial-cadherin disruption, both of which play critical roles in modulating endothelial permeability. Notably, microvascular leakage in the retina of diabetic mice was successfully inhibited by intravitreal Dammarenediol II injection.
CONCLUSIONS:
Our results suggest that the natural drug Dammarenediol II may have the ability to prevent diabetic microvascular complications, including diabetic retinopathy.
Plant Sci. 2015 Oct;239:106-14.
Production of dammarane-type sapogenins in rice by expressing the dammarenediol-II synthase gene from Panax ginseng C.A. Mey.[Pubmed:
26398795 ]
Ginsenosides are the main active ingredients in Chinese medicinal ginseng; 2,3-oxidosqualene is a precursor metabolite to ginsenosides that is present in rice. Because rice lacks a key rate-limiting enzyme (Dammarenediol II synthase, DS), rice cannot synthesize dammarane-type ginsenosides.
METHODS AND RESULTS:
In this study, the ginseng (Panax ginseng CA Mey.) DS gene (GenBank: AB265170.1) was transformed into rice using agrobacterium, and 64 rice transgenic plants were produced. The Transfer-DNA (T-DNA) insertion sites in homozygous lines of the T2 generation were determined by using high-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR) and differed in all tested lines. One to two copies of the T-DNA were present in each transformant, and real-time PCR and Western blotting showed that the transformed DS gene could be transcribed and highly expressed. High performance liquid chromatography (HPLC) analysis showed that the dammarane-type sapogenin 20(S)-protopanaxadiol (PPD) content was 0.35-0.59 mg/g dw and the dammarane-type sapogenin 20(S)-protopanaxatriol (PPT) content was 0.23-0.43 mg/g dw in the transgenic rice. LC/MS analysis confirmed production of PPD and PPT.
CONCLUSIONS:
These results indicate that a new "ginseng rice" germplasm containing dammarane-type sapogenins has been successfully developed by transforming the ginseng DS gene into rice.
Acta Crystallogr Sect E Struct Rep Online. 2012 Nov 1;68(Pt 11):o3089-90.
3-epi-Dammarenediol II 1.075 hydrate: a dammarane triterpene from the bark of Aglaia eximia.[Pubmed:
23284420]
METHODS AND RESULTS:
The title dammarane tritepene, 3α,20(S)-dihy-droxy-dammar-24-ene, which crystallized out in a hydrated form, C(30)H(52)O(2).1.075H(2)O, was isolated from the Aglaia eximia bark. The three cyclo-hexane rings adopt chair conformations. The cyclo-pentane has an envelope conformation with the quaternary C at position 14 as the flap atom with the maximum deviation of 0.288 (2) Å. The methyl-heptene side chain is disordered over two positions with 0.505 (1):0.495 (1) site occupancies and is axially attached with an (+)-syn-clinal conformation. The hydroxyl group at position 3 of dammarane is in a different conformation to the corresponding hydroxyl in Dammarenediol II.
CONCLUSIONS:
In the crystal, the dammarane and water mol-ecules are linked by O(Dammarane)-H⋯O(water) and O(water)-H⋯O(Dammarane) hydrogen bonds into a three-dimensional network.