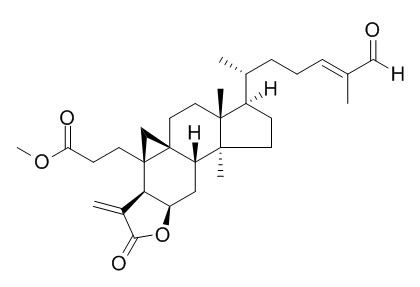
Coronalolide methyl ester
Coronalolide methyl ester displays significant anti-HIV activities in the HIV-1RT assay. It and coronalolide show broad cytotoxic activity against a panel of human cancer cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2024, 15:1439079.
Horticulturae2023, 9(2), 213.
Earth Environ. Sci. 2021, 905:012080.
University of Stuttgart2021, 11682.
J Sep Sci.2018, 41(7):1682-1690
Biomed Pharmacother.2021, 137:111362.
Nutrients.2024, 16(15):2518.
Fitoterapia.2022, 105141.
Int J Mol Sci.2024, 25(5):2914.
Patanjali Research Foundation2024, ssrn.4807357
Related and Featured Products
Tetrahedron,2004, 60(7):1517-23.
Cytotoxic and anti-HIV-1 constituents from leaves and twigs of Gardenia tubifera[Reference:
WebLink]
METHODS AND RESULTS:
Two new natural cycloartanes, tubiferolide methyl ester (1) and tubiferaoctanolide (2), together with the known coronalolide (3) and Coronalolide methyl ester (4) have been isolated from leaves and twigs of Gardenia tubifera. In addition, a new flavone 5,3′,5′-trihydroxy-7,4′-dimethoxyflavone (5), five known flavones 6–10 and hexacosyl 4′-hydroxy-trans-cinnamate (11) were also obtained from the same source. The structures were assigned on the basis of spectroscopic methods.
CONCLUSIONS:
Compounds 3, 7, 9, and 10 showed significant cytotoxic activities only in P-388 cell line. Compound 1 was cytotoxic against P-388, KB, Col-2 and Lu-1, while 4 was active in P-388 and BCA-1. Compounds 3 and 4 displayed significant anti-HIV activities in the HIV-1RT assay; compound 7 showed moderate activity in this assay. Compounds 5–10 were also found to be active in the ΔTat/RevMC 99 syncytium assay.
Tetrahedron,1997,28(18):529-38.
Novel cytotoxic ring-a seco-cycloartane triterpenes from Gardenia coronaria and G. sootepensis[Reference:
WebLink]
METHODS AND RESULTS:
Coronalolide methyl ester (1), coronalolide (2), and coronalolic acid (3) were isolated from the leaves and/or stems of Gardenia coronaria. A further compound, methyl coronalolate acetate (4), was purified from the stems after methylation. The novel compounds 1–4 have the rare ring-A seco-cycloartane carbon skeleton and their structures were assigned on the basis of spectral data and molecular modeling, as well as X-ray crystallography performed on 1.
CONCLUSIONS:
Compounds 1 and 2 were also isolated from the leaves of Gardenia sootepensis and showed broad cytotoxic activity when evaluated against a panel of human cancer cell lines.