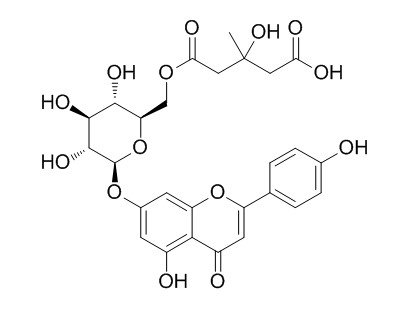
Chamaemeloside
Chamaemeloside has hypoglycaemic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Cell Physiol Biochem.2019, 52(6):1255-1266
J Pharmaceut Biomed2020, 182:113110
Buildings2023, 13(5), 1112.
Int J Mol Sci.2023, 24(8):7045.
Eur J Pharmacol.2023, 951:175770.
STAR Protoc.2024, 5(2):102990.
Int J Mol Sci.2020, 21(8):2790.
Molecules.2020, 25(20):4851.
Pharm Biol.2021, 59(1):134-145.
Prev Nutr Food Sci.2025, 30(1):92-100.
Related and Featured Products
Planta Medica, 1998, 64(7):612-614.
Hypoglycaemic activity of an HMG-containing flavonoid glucoside, chamaemeloside, from Chamaemelum nobile.[Reference:
WebLink]
METHODS AND RESULTS:
The 3-hydroxy-3-methylglutaric acid (HMG) containing flavonoid glucoside Chamaemeloside, has been determined to have in vivo hypoglycaemic activity comparable to that of free HMG. An improved isolation scheme for obtaining Chamaemeloside from Chamaemelum nobile is presented.
Journal of Separation Science, 01 Sep 2014, 37(19):2797-2804.
Quantitative determination of seven chemical constituents and chemo‐type differentiation of chamomiles using high‐performance thin‐layer chromatography.[Reference:
WebLink]
METHODS AND RESULTS:
A simple and rapid high‐performance thin‐layer chromatographic method was developed for the separation and determination of six flavonoids (rutin, luteolin‐7‐O‐β‐glucoside, Chamaemeloside, apigenin‐7‐O‐β‐glucoside, luteolin, apigenin) and one coumarin, umbelliferone from chamomile plant samples and dietary supplements. The separation was achieved on amino silica stationary phase using dichloromethane/acetonitrile/ethyl formate/glacial acetic acid/formic acid (11:2.5:3:1.25:1.25 v/v/v/v/v) as the mobile phase. The quantitation of each compound was carried out using densitometric reflection/absorption mode at their respective absorbance maxima after postchromatographic derivatization using natural products reagent (1% w/v methanolic solution of diphenylboric acid‐β‐ethylamino ester). The method was validated for specificity, limits of detection and quantification, precision (intra‐ and interday) and accuracy. The limits of detection and quantification were found to be in the range from 6–18 and 16–55 ng/band for six flavonoids and one coumarin, respectively. The intra‐ and interday precision was found to be <5% RSD and recovery of all the compounds was >90%.
CONCLUSIONS:
The data acquired from high‐performance thin‐layer chromatography was processed by principal component analysis using XLSTAT statistical software. Application of principal component analysis and agglomerative hierarchial clustering was successfully able to differentiate two chamomiles (German and Roman) and Chrysanthemum.