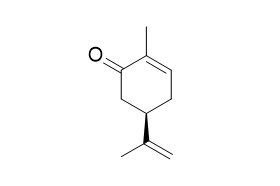
(-)-Carvone
(-)-Carvone has antinociceptive activity associated with decreased peripheral nerve excitability. (-)-Carvone shows fungicidal activity, it could be effective biocontrol agents against S. oryzae and T. castaneum.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
EXCLI J.2023, 22:482-498.
Int J Biol Macromol.2018, 112:1093-1103
Plant Physiol Biochem.2021, 160:166-174.
Foods.2024, 13(19):3092.
Arch Pharm Res.2015, 38(6):1080-9
Foods2023, 12(23), 4342.
Applied Biological Chemistry2022, 65(77).
J Sep Sci.2022, 45(18):3556-3566.
Food Res Int.2022, 157:111207.
J Plant Biochem.Biotech.2024, 33:353-366.
Related and Featured Products
Plant Disease, 2007, 91(1):30-35.
Fungicidal Activity of Plant Volatile Compounds for Controlling Monilinia laxa in Stone Fruit .[Reference:
WebLink]
Nine plant-volatile compounds were tested for their activity against Monilinia laxa, the cause of brown rot in stone fruit.
METHODS AND RESULTS:
In vitro trials on conidial germination and mycelial growth showed a consistent fungicidal activity of trans-2-hexenal, carvacrol, and citral, whereas trans-cinnamaldehyde, hexanal, (-)-Carvone, eugenol, 2-nonanone, and p-anisaldehyde exhibited a progressively lower inhibition. The best inhibitor of conidial germination was trans-2-hexenal (effective dose for 50 and 90% inhibition [ED50 and ED95] = 7.53 and 9.4 μl/liter, respectively; minimal inhibitory concentration [MIC] = 12.3 μl/liter], whereas carvacrol was the best inhibitor of mycelial growth (ED50 and ED95 = 2 and 3.4 μl/liter, respectively; MIC = 6.1 μl/liter). The three most active compounds in in vitro studies also were tested in vivo as postharvest biofumigants.
CONCLUSIONS:
The best control of brown rot was with trans-2-hexenal (efficacy ranging from 46.2 to 80.3%, depending on cultivar), whereas citral and carvacrol resulted in a lower efficacy of 40 and 32.9%, respectively. Fumigation with trans-2-hexenal at concentrations that stopped decay did not cause any visible disorders to plum, whereas it was phytotoxic to apricot, peach, and nectarine and produced off-odors or off-flavors in all species of stone fruit tested.
Journal of Chemical Ecology, 2009, 35(5):518-525.
Fumigant and Contact Toxicities of Monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their Inhibitory Effects on Acetylcholinesterase Activity.[Reference:
WebLink]
METHODS AND RESULTS:
A comparative study was conducted to assess the contact and fumigant toxicities of eleven monoterpenes on two important stored products insects--, Sitophilus oryzae, the rice weevil, and Tribolium castaneum, the rust red flour beetle. The monoterpenes included: camphene, (+)-camphor, (-)-Carvone, 1-8-cineole, cuminaldehyde, (L: )-fenchone, geraniol, (-)-limonene, (-)-linalool, (-)-menthol, and myrcene. The inhibitory effect of these compounds on acetylcholinesterase (AChE) activity also was examined to explore their possible mode(s) of toxic action. Although most of the compounds were toxic to S. oryzae and T. castaneum, their toxicity varied with insect species and with the bioassay test. In contact toxicity assays, (-)-Carvone, geraniol, and cuminaldehyde showed the highest toxicity against S. oryzae with LC(50) values of 28.17, 28.76, and 42.08 microg/cm(2), respectively. (-)-Carvone (LC(50) = 19.80 microg/cm(2)) was the most effective compound against T. castaneum, followed by cuminaldehyde (LC(50) = 32.59 microg/cm(2)). In contrast, camphene, (+)-camphor, 1-8-cineole, and myrcene had weak activity against both insects (i.e., LC(50) values above 500 microg/cm(2)). In fumigant toxicity assays, 1-8-cineole was the most effective against S. oryzae and T. castaneum (LC(50) = 14.19 and 17.16 mg/l, respectively).
Structure-toxicity investigations revealed that (-)-Carvone--, a ketone--, had the highest contact toxicity against the both insects. 1-8-Cineole--, an ether--, was the most potent fumigant against both insects. In vitro inhibition studies of AChE from adults of S. oryzae showed that cuminaldehyde most effectively inhibited enzyme activity at the two tested concentrations (0.01 and 0.05 M) followed by 1-8-cineole, (-)-limonene, and (L)-fenchone. 1-8-Cineole was the most potent inhibitor of AChE activity from T. castaneum larvae followed by (-)-Carvone and (-)-limonene.
CONCLUSIONS:
The results of the present study indicate that (-)-Carvone, 1,8-cineole, cuminaldehyde, (L)-fenchone, and (-)-limonene could be effective biocontrol agents against S. oryzae and T. castaneum.
Biological & Pharmaceutical Bulletin, 2008, 31(5):1017-20.
Antinociceptive activity of (-)-carvone: evidence of association with decreased peripheral nerve excitability.[Reference:
WebLink]
(-)-Carvone is a monoterpene ketone that is the main active component of Mentha plant species like Mentha spicata. This study aimed to investigate the antinociceptive activity of (-)-Carvone using different experimental models of pain and to investigate whether such effects might be involved in the nervous excitability elicited by others monoterpenes.
METHODS AND RESULTS:
In the acetic acid-induced writhing test, we observed that (-)-Carvone-treated mice exhibited a significant decrease in the number of writhes when 100 and 200 mg/kg was administered. It was also demonstrated that (-)-Carvone inhibited the licking response of the injected paw when 100 and 200 mg/kg was administered (i.p.) to mice in the first and second phases of the formalin test. Since naloxone (5 mg/kg, s.c.), an opioid antagonist, showed no influence on the antinociceptive action of (-)-Carvone (100 mg/kg), this suggested nonparticipation of the opioid system in the modulation of pain induced by (-)-Carvone. Such results were unlikely to be provoked by motor abnormality, since (-)-Carvone-treated mice did not exhibit any performance alteration on the Rota-rod apparatus.
Because the antinociceptive effects could be associated with neuronal excitability inhibition, we performed the single sucrose gap technique and observed that (-)-Carvone (10 mM) was able to reduce the excitability of the isolated sciatic nerve through a diminution of the compound action potential amplitude by about 50% from control recordings.
CONCLUSIONS:
We conclude that (-)-Carvone has antinociceptive activity associated with decreased peripheral nerve excitability.