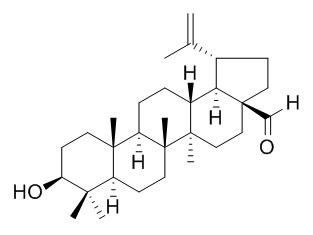
Betulinaldehyde
Betulinaldehyde exhibits antimicrobial activity against reference strains of methicillin-resistant and methicillin-sensitive S. aureus. It shows antimycobacterial activity against with values of 25 microg/ml.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Natural Product Communications2020, doi: 10.1177.
Chem Biol Interact.2016, 258:59-68
ACS Omega.2024, 9(12):14356-14367.
Antioxidants (Basel).2020, 9(2): E119
Environ Toxicol.2023, 23929.
Rec. Nat. Prod.2024, 18:4,405-418.
Journal of Natural Remedies2024, 24(3):555¨C575.
Oncology Letters2018, 4690-4696
J Microbiol Biotechnol.2023, 33(10):1317-1328.
Int J Mol Sci.2022, 23(24):16000.
Related and Featured Products
Chem Pharm Bull (Tokyo). 2006 Apr;54(4):535-7.
Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana.[Pubmed:
16595959]
One new and eight known ceanothane- and lupane-type triterpenes were isolated from the root bark of Ziziphus cambodiana PIERRE (Rhamnaceae).
METHODS AND RESULTS:
Based on spectral analyses, the structure of the new compound was elucidated as 3-O-(4-hydroxy-3-methoxybenzoyl)ceanothic acid (3-O-vanillylceanothic acid) (1), while the known compounds were identified as lupeol (2), Betulinaldehyde (3), betulinic acid (4), 2-O-E-p-coumaroyl alphitolic acid (5), alphitolic acid (6), zizyberanalic acid (7), zizyberenalic acid (8) and ceanothic acid (9). Compounds 1, 5 and 8 exhibited significant in vitro antiplasmodial activity against the parasite Plasmodium falciparum, with inhibitory concentration (IC50) values of 3.7, 0.9 and 3.0 microg/ml, respectively. Compounds 1 and 3-8 showed antimycobacterial activity against Mycobacterium tuberculosis with respective MIC values of 25, 25, 25, 12.5, 50, 50 and 100 microg/ml.
Fitoterapia. 2014 Apr;94:48-54.
Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus.[Pubmed:
24508863]
The evolution of antibiotic resistance in Staphylococcus aureus showed that there is no long-lasting remedy against this pathogen. The limited number of antibacterial classes and the common occurrence of cross-resistance within and between classes reinforce the urgent need to discover new compounds targeting novel cellular functions not yet targeted by currently used drugs. One of the experimental approaches used to discover novel antibacterials and their in vitro targets is natural product screening.
METHODS AND RESULTS:
Three known pentacyclic triterpenoids were isolated for the first time from the bark of Callicarpa farinosa Roxb. (Verbenaceae) and identified as α-amyrin [3β-hydroxy-urs-12-en-3-ol], betulinic acid [3β-hydroxy-20(29)-lupaene-28-oic acid], and Betulinaldehyde [3β-hydroxy-20(29)-lupen-28-al]. These compounds exhibited antimicrobial activities against reference and clinical strains of methicillin-resistant (MRSA) and methicillin-sensitive S. aureus (MSSA), with minimum inhibitory concentration (MIC) ranging from 2 to 512 μg/mL. From the genome-wide transcriptomic analysis to elucidate the antimicrobial effects of these compounds, multiple novel cellular targets in cell division, two-component system, ABC transporters, fatty acid biosynthesis, peptidoglycan biosynthesis, aminoacyl-tRNA synthetases, ribosomes and β-lactam resistance pathways are affected, resulting in destabilization of the bacterial cell membrane, halt in protein synthesis, and inhibition of cell growth that eventually lead to cell death.
CONCLUSIONS:
The novel targets in these essential pathways could be further explored in the development of therapeutic compounds for the treatment of S. aureus infections and help mitigate resistance development due to target alterations.