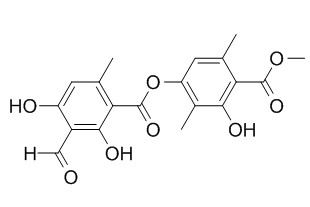
Atranorin
Atranorin shows significant antinociceptive and antiinflammatory activities, it has a relevant redox-active action, acting as a pro-oxidant or antioxidant agent depending on the radical, also, it will exert cytoprotective effects on cells under oxidative stress induced by H(2)O(2). Atranorin has suppression of viability and proliferation in tumour cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Mol Histol.2019, 50(4):343-354
Natural Product Communications2022, 7(3):1-7.
Pharmaceuticals (Basel).2024, 17(8):988.
Appl Microbiol Biotechnol.2024, 108(1):207.
Univerzita Karlova2022, 173245.
Int J Mol Sci.2024, 25(12):6456.
Phytomedicine.2015, 22(14):1262-8
Acta Biochim Pol.2015, 62(2):253-8
J Cell Mol Med.2023, jcmm.18071.
NanoBioScience2024, v13:3:115.
Related and Featured Products
Nat Prod Commun. 2014 Apr;9(4):503-4.
Clastogenic effect of atranorin, evernic acid, and usnic acid on human lymphocytes.[Pubmed:
24868868]
METHODS AND RESULTS:
Three lichen secondary metabolites Atranorin (1), evernic acid (2), and usnic acid (3), were evaluated for their in vitro clastogenic and antiproliferative effects on human lymphocytes using the cytochalasin-B blocked micronucleus (CBMN) assay at concentrations of 2 microg/mL, 4 microg/mL and 6 microg/mL of final culture solution. Atranorin at concentrations of 2 microg/mL and 4 microg/mL decreasing the frequency of MN only for 11.1% and 1.8%, while in concentration of 6 microg/mL increases the frequency of MN for 9.6 %.
CONCLUSIONS:
The comparable CBPI values of the investigated compounds and control suggested that they did not show a statistically significant inhibitory effect on lymphocyte cell proliferation at applied concentrations.
Toxicol In Vitro. 2011 Mar;25(2):462-8.
Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite.[Pubmed:
21111802]
Atranorin (ATR) is a lichenic secondary metabolite with potential uses in pharmacology. Antinociceptive and antiinflammatory actions have been reported, and the use of Atranorin-enriched lichen extracts in folk medicine is widespread. Nonetheless, very few data on Atranorin biological actions are available.
METHODS AND RESULTS:
Here, we evaluated free radical scavenging activities and antioxidant potential of Atranorin using various in vitro assays for scavenging activity against hydroxyl radicals, hydrogen peroxide, superoxide radicals, and nitric oxide. The total reactive antioxidant potential (TRAP) and total antioxidant reactivity (TAR) indexes and in vitro lipoperoxidation were also evaluated. Besides, we determined the cytoprotective effect of ATR on H(2)O(2)-challenged SH-SY5Y cells by the MTT assay. Atranorin exerts differential effects towards reactive species production, enhancing hydrogen peroxide and nitric oxide production and acting as a superoxide scavenger; no activity toward hydroxyl radical production/scavenging was observed. Besides, TRAP/TAR analysis indicated that Atranorin acts as a general antioxidant, although it demonstrated to enhance peroxyl radical-induced lipoperoxidation in vitro. Atranorin was not cytotoxic, and also protected SH-SY5Y cells against H(2)O(2)-induced cell viability impairment.
CONCLUSIONS:
Our results suggest that Atranorin has a relevant redox-active action, acting as a pro-oxidant or antioxidant agent depending on the radical. Also, it will exert cytoprotective effects on cells under oxidative stress induced by H(2)O(2).
Toxicol In Vitro. 2011 Feb;25(1):37-44.
Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid.[Pubmed:
20837130]
One of the ways for searching for potentially new anti-cancer drugs is the testing of various naturally synthesized compounds. Lichens are a source of unique chemical agents of which some have already been proved to be effective against various cancer in vitro models.
METHODS AND RESULTS:
Our study reports on the sensitivity of up to nine human cancer cell lines (A2780, HeLa, MCF-7, SK-BR-3, HT-29, HCT-116 p53(+/+), HCT-116 p53(-/-), HL-60 and Jurkat) to the anti-proliferative/cytotoxic effects of four typical secondary metabolites of lichens (parietin, Atranorin, usnic acid and gyrophoric acid). Variations in the dynamics of tumour cell line populations were evaluated by the MTT, clonogenic and viability assays, cell proliferation and detachment, cell cycle transition and apoptotic nuclear morphology, thereby confirming their concentration- and time-dependent cytotoxicity. However, in comparison with parietin and gyrophoric acid, the suppression of viability and cell proliferation by usnic acid or Atranorin was found to be more efficient at equitoxic doses and correlated more strongly with an increased number of floating cells or a higher apoptotic index. Moreover, the analysis of cell cycle distribution also revealed an accumulation of cells in S-phase.
CONCLUSIONS:
This study has confirmed a differential sensitivity of cancer cell lines to lichen secondary metabolites.
Z Naturforsch C. 2010 Sep-Oct;65(9-10):551-61.
Antinociceptive activity of atranorin in mice orofacial nociception tests.[Pubmed:
21138055]
Physicochemical characterization and antinociceptive and anti-inflammatory activities of Atranorin (AT) extracted from Cladina kalbii Ahti in formalin- and capsaicin-induced orofacial pain and anti-inflammatory tests in rodents were studied. Physicochemical characterization showed that AT has the general formula C19H18O8.
METHODS AND RESULTS:
Male Swiss mice were pretreated with Atranorin (100, 200, and 400 mg/kg, i.p.), morphine (3 mg/kg, i.p.), or vehicle (0.9% saline with two drops of 0.2% Tween 80) before formalin (20 microl, 2%) or capsaicin (20 microl, 2.5 microg) were injected into the right vibrissa. Our results showed that i.p. treatment with Atranorin displayed marked inhibitory effects in different orofacial pain tests in mice. Atranorin (400 mg/kg, i.p.) was effective in reducing the nociceptive face-rubbing behavioural response in both phases of the formalin test, which was also naloxone-sensitive. Additionally, Atranorin produced a significant antinociceptive effect at all doses in the capsaicin test. Such results were unlikely to be provoked by motor abnormality, since Atranorin-treated mice exhibited no performance alteration on the rota rod apparatus. Atranorin exhibited significant anti-inflammatory activity in the acute model of inflammation (leukocyte migration to the peritoneal cavity), carrageenan- and arachidonic acid-induced hind paw edema in rats. Additionally, Atranorin exhibited a dose-dependent antioxidant activity in vitro, as assessed by total radical-trapping antioxidant parameter and total antioxidant reactivity assays.
CONCLUSIONS:
All these findings suggest that Atranorin might represent an important tool for the management of orofacial pain and/or inflammatory disorders.