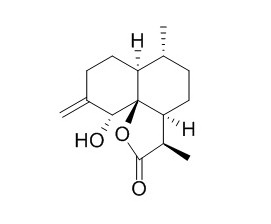
Arteannuin L
Arteannuin can degrade the level of TNF-α in rats with ostarthritis,and effectively inhibit the inflammation.It also has antimalarial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Neurochem Int.2023, 167:105537.
J Agric Food Chem.2020, 68(51):15164-15175
Eur Rev Med Pharmacol Sci.2020, 24(9):5127-5139.
JAOCS2021, 98(7):779-794.
Asian J Beauty Cosmetol2020, 18(3): 265-272.
Antioxidants (Basel).2023, 12(1):189.
J Vet Sci.2020, 21(3):e39.
Allergol Immunopathol (Madr).2022, 1;50(4):23-30.
Applied Biological Chemistry2023, 66(58):112.
Ecol Evol.2022, 12(11):e9459.
Related and Featured Products
Journal of Xinxiang Medical College,2012, 29(11): 813-4.
Effect of arteannuin on expression of tumor necrosis factor-α in rats with ostarthritis.[Reference:
WebLink]
To observe the effect of arteannuin on the level of tumor necrosis factor-α(TNF-α) in rats with ostarthritis.
METHODS AND RESULTS:
Thirty-two rats with ostarthritis were randomly divided into control group,high-dose arteannuin group,media-dose arteannuin group and low-dose arteannuin group,eight rats in each group.The rats in control group were given with saline 20 mL by gavage,once a day.The rats in high-dose arteannuin group,media-dose arteannuin group and low-dose arteannuin group were given with arteannuin 400,300 and 200 mg·kg-1 respectively by gavage,once a day for seven weeks(the arteannuin was dissolved in 20 mL saline).The level of TNF-α was detected by radioimmunoassay(RIA),and the expressions of TNF-α mRNA and protein were detected by reverse transcription-polymerase chain reaction and Western blotting.The levels of serum TNF-α in high-dose,media-dose and low-dose arteannuin groups were decreased significantly than that of control group(P0.05).The level of serum TNF-α in low-dose arteannuin group was higher significantly than those in high-dose and media-dose arteannuin group(P0.05),and the level of serum TNF-α in the media-dose group was higher significantly than that in high-dose arteannuin group(P0.05).There was no statistically significant difference in the expression of TNF-α mRNA between the media-dose and low-dose arteannuin group(P0.05).The expression of TNF-α mRNA in high-dose arteannuin group was lower significantly than those in media-dose and low-dose arteannuin group(P0.05).The expression of TNF-α protein in high-dose and media-dose arteannuin group were lower significantly than those in control group and low-dose arteannuin group(P0.05),but there was no statistically significant difference in the expression of TNF-α protein between media-dose and high-dose arteannuin group(P0.05).
CONCLUSIONS:
Arteannuin can degrade the level of TNF-α in rats with ostarthritis,and effectively inhibit the inflammation.
Tetrahedron.2001 Oct;57(40):8481–8493.
Structure elucidation of arteannuin O, a novel cadinane diol from Artemisia annua, and the synthesis of arteannuins K, L, M and O.[Reference:
WebLink]
The novel cadinane diol, arteannuin O (1), has been obtained from Artemisia annua and its structure has been established by 2D NMR and X-ray crystallography.
METHODS AND RESULTS:
A reconstructive synthesis of arteannuin O from artemisinin is described, which also yields the natural products arteannuin K and Arteannuin L. Mechanistic considerations have led to the conclusion that the stereochemistry of the 5-hydroxyl group was wrongly assigned when arteannuin K, Arteannuin L and M were first reported as natural products.
CONCLUSIONS:
This was confirmed by derivatization of synthetic arteannuin K, Arteannuin L and M as their Mosher esters.
Acta Chimica Sinica,1989, 47(4):340-4.
Studies on the Structure and Synthesis of Arteannuin and Related Compound XXII. The Regioselective Synthesis of Arteannuin D[Reference:
WebLink]
Arteannuin D (3) coexists with arteannuin (1), which in an antimalarial principle isolated from Chinese medicinal herb Artemisia annua L.
METHODS AND RESULTS:
In this paper the regioselective synthesis of 3 is reported. The aldehyde-ketone 8 obtained from arteannuinic acid (7), was treated with 1 eq trimethylorthoformate in methanol in the presence of a catalytic amount of p-TsOH to provide 9 and 10 in 86% yield in the ratio of 1 to 1, and with 2 eq. of the orthoformate under the same condition to give only 9 in 88% yield. However, pyrolysis of 9 in xylene did not give the desired intermediate 5, but gave 11 and 12 in the yields of 34% and 30%, respectively. Another approach to the synthesis of 3 is to use enol-silyl ether 13 obtained from 8 as a key intermediate through the following sequence of reactions: 8→10→12→13 in 28% overall yield. Trimethylsilyl enol ether 13 was hydroxylated with N-methyl morpholine N-oxide (NMMNO) and a catalytic amount of OsO_4 to give the 3-OH product 14 regioselectively in 52% yield. While the compound 12 was hydroxylated with OsO_4 and NMMNO, followed by cyolization with 10% K_2CO_3 to produce deoxyarteannuin (2) in 72% yield. After protection of 3-OH of 14 by acetylation, it was hydroxylated with a stoichiometric amount of Os0_4 followed by cyclization with 10% K_2CO_3 to give a mixture of 3 and its 3-OH epimer 15 in 59% yield, which after column chromatography gave 3, m. p. 190--192℃, whose spectroscopic data were identical with those reported in the literature.