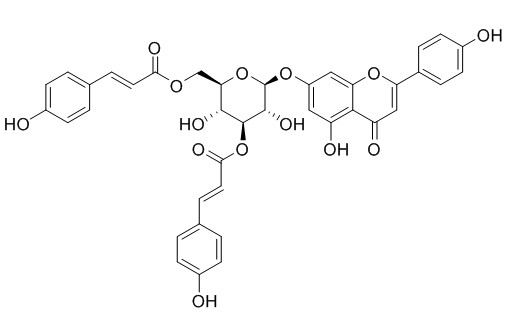
Anisofolin A
Anisofolin A has promising antimalarial activity (IC50 4.39 ± 0.25 uM). It has promising antimycobacterium activity [IC50 4.50 ± 0.75 uM (3.31 ug/mL)] against M. tuberculosis H37Ra and at 100 ug/mL, shows 55.6 % inhibition of M. bovis.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2019, 20(23):E6071
Food Chem.2024, 460(Pt 1):140472.
Int. J of Herbal Med.2023, 11(1): 06-14
RSC Adv.2018, 32621-32636
Animals (Basel).2024, 14(20):2990.
Horticulture Research2023, uhad259
Food Chem Toxicol.2023, 176:113802.
Sci Rep.2024, 14(1):26330.
Journal of Functional Foods2017, 30:30-38
VNU Journal of Science2023, No. 20.
Related and Featured Products
Planta Med Lett 2015; 2(01): e35-e38
Leucas mollissima, a Source of Bioactive Compounds with Antimalarial and Antimycobacterium Activities[Reference:
WebLink]
METHODS AND RESULTS:
A phytochemical investigation of the acetone extract from the aerial parts of Leucas mollissima afforded one new (−)epi-marmelo lactone, (2 S, 4R, 6 S)-2,6-dimethyl-6 hydroxy-7-ene-4-olide (1), along with five known compounds, schensianol A (2), vanillin (3), β-hydroxy propiovanillone (4), lanost-9(11),25-diene-3β,24β-diol (5), and lanost-9(11),23E(24)-diene-3β,25-diol (6). Similarly, an investigation of the methanol extract of the aerial parts of L. mollissima resulted in the isolation of three known compounds, (+)-syringaresinol (7), Anisofolin A (8), and apigenin 7-O-β-D(− 6′′-p-E-coumaroyl)-glucoside (9). Structure elucidation of the isolated compounds was carried out using detailed analysis of 1D and 2D nuclear magnetic resonance. All compounds were evaluated for antimalarial activity against Plasmodium falciparum (3D7) and for antimycobacterium activity against Mycobacterium tuberculosis H37Ra and Mycobacterium bovis.
CONCLUSIONS:
Compound 8 was found to have promising antimalarial activity (IC50 4.39 ± 0.25 μM), promising antimycobacterium activity [IC50 4.50 ± 0.75 μM (3.31 μg/mL)] against M. tuberculosis H37Ra and at 100 μg/mL, showed 55.6 % inhibition of M. bovis. Compound 9 showed moderate inhibition of P. falciparum growth (35 % inhibition at 10 μM) with respect to the positive control atovaquone and 67.4 % inhibition against M. bovis at 100 μg/mL with respect to the positive control rifampicin.
Chem Pharm Bull (Tokyo). 2006 Oct;54(10):1370-9.
Studies on Nepalese crude drugs. XXIX. Chemical constituents of Dronapuspi, the whole herb of Leucas cephalotes SPRENG.[Pubmed:
17015972]
METHODS AND RESULTS:
From the whole herb of Leucas cephalotes SPRENG., new labdane-, norlabdane- and abietane-type diterpenes named leucasdins A (1), B (2) and C (3), respectively, and two protostane-type triterpenes named leucastrins A (4) and B (5) were isolated, together with a known triterpene, oleanolic acid, five sterols, 7-oxositosterol, 7-oxostigmasterol, 7alpha-hydroxysitosterol, 7alpha-hydroxystigmasterol and stigmasterol, and eight flavones, 5-hydroxy-7,4'-dimethoxyflavone, pillion, gonzalitosin I, tricin, cosmosin, apigenin 7-O-beta-D-(6-O-p-coumaroyl)glucopyranoside, Anisofolin A and luteolin 4'-O-beta-D-glucuronopyranoside.
CONCLUSIONS:
The structures of 1--5 were determined as (3S,6R,8R,9R,13S,16S)-9,13,15,16-bisepoxy-3,16-diacetoxy-6-formyloxylabdane, (3S,6R)-3-acetoxy-6-formyloxy-iso-ambreinolide, (4R,9S,12R,13R)-12,13-dihydroxyabiet-7-en-18-oic acid, (3S,17S,20S,24S)-3,20-dihydroxy-24-methylprotost-25-en, and (3S,17S,20S,24S)-3,20,24-trihydroxyprotost-25-en respectively, based on spectral and chemical data.