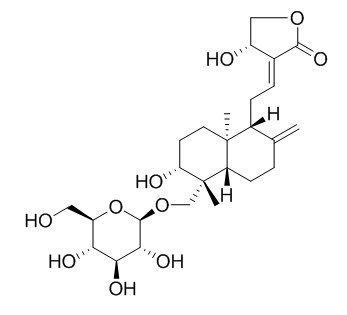
Andrographiside
Andrographiside shows protective effects on hepatotoxicity induced in mice by carbon tetrachloride or tert-butylhydroperoxide (tBHP) intoxication, the effects could be due to its glucoside groups which may act as strong antioxidants.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2024, 15:1455805.
University of Central Lancashire2017, 20472
Phytother Res.2018, 32(12):2551-2559
ARPN Journal of Eng.& Applied Sci.2016, 2199-2204
Foods.2022, 11(6):882.
Pharmaceutics.2021, 13(7):1028.
Biomed Chromatogr.2016, 30(10):1573-81
Biomed Chromatogr.2019, 8:e4774
Analytical sci. & Tech2016, 186-193
Pharmacognosy Magazine2024, 20(2):632-645.
Related and Featured Products
Biochemical Pharmacology.1993 Jul;46(1):182-185.
Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata.[Reference:
WebLink]
The diterpenes andrographolide (I), Andrographiside (II) and neoandrographolide (III) isolated from Andrographis paniculata were investigated for their protective effects on hepatotoxicity induced in mice by carbon tetrachloride or tert-butylhydroperoxide (tBHP) intoxication.
METHODS AND RESULTS:
Pretreatment of mice with the diterpenes (I, II & III; 100 mg/kg, i.p.) for 3 consecutive days produced significant reduction in malondialdehyde formation, reduced glutathione (GSH) depletion and enzymatic leakage of glutamic-pyruvate transaminase (GPT) and alkaline phosphatase (AP) in either group of the toxintreated animals.
CONCLUSIONS:
A comparison with the known hepatoprotective agent silymarin revealed that I exhibited a lower protective potential than Andrographiside and III, which were as effective as silymarin with respect to their effects on the formation of the degradation products of lipid peroxidation and release of GPT and AP in the serum. GSH status was returned to normal only by III. The greater protective activity of Andrographiside and III could be due to their glucoside groups which may act as strong antioxidants.
Journal of Molecular Structure.2010 Feb25; 965(1–3):45–49.
Crystal structure and DFT calculations of andrographiside.[Reference:
WebLink]
METHODS AND RESULTS:
Crystal and molecular structure of a labdane diterpenoid glucoside, Andrographiside (1) is determined from 2D-NMR and X-ray diffraction data. The 2D-NMR study indicates that the carbohydrate moiety is in β-linkage and the sugar moiety is linked to C-19 of the aglycon. These observations are further confirmed from the X-ray diffraction studies. Both the six-membered rings are in chair conformation whereas the glucose ring adopts a twist-boat conformation. The molecular geometries and electronic structure of Andrographiside were calculated at the DFT level using the hybrid exchange–correlation functional, BLYP, PW91 and PBE.
Nat Prod Commun. 2013 Mar;8(3):333-4.
Andrographidine G, a new flavone glucoside from Andrographis paniculata.[Pubmed:
23678804]
A new flavone glucoside, andrographidine G (1), was isolated from Andrographis paniculata together with 13 known compounds, including flavonoids, diterpenoids, and iridoids.
METHODS AND RESULTS:
The structure of 1 was established by spectroscopic and spectrometric techniques, including HR-ESI-TOF-MS, 1D and 2D NMR, and chemical methods. The known compounds were identified as andrographidine A (2), (2R)-5-hydroxy-7,8-dimethoxyflavanone-5-O-beta-D-glucopyranoside (3), acanthoside B (4), neoAndrographiside (5), andropanoside (6), Andrographiside (7), andrographolide (8), 14-deoxy-11,12-didehydroAndrographiside (9), 14-deoxy-11,12-didehydroandrographolide (10), procumbide (11), procumboside (12), 6-epi-8-O-acetylharpagide (13), and curvifloruside F (14).