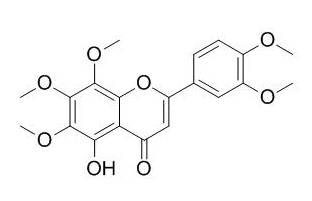
5-O-Demethylnobiletin
5-O-Demethylnobiletin has anti-inflammatory activity, it may act through a direct inhibition of 5-LOX, without affecting the expression of COX-2.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2019, 20(21):E5488
Hum Exp Toxicol.2022, 41:9603271221143713.
J Sep Sci.2020, 43(22):4148-4161.
Biomedicines.2022, 10(5):1170
Phytomedicine.2020, 79, 153351
An Acad Bras Cienc.2023, 95(3):e20220672
Antioxidants (Basel).2023, 12(12):2131.
Food Chem.2018, 262:78-85
Chulalongkorn University2024, 4761190
Sci Rep. 2017, 17332(7)
Related and Featured Products
Planta Med. 2006 Feb;72(2):136-42.
Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum.[Pubmed:
16491449 ]
METHODS AND RESULTS:
We have studied the effect of 5-O-Demethylnobiletin ( 1) on both the inflammation of mouse ears induced by repeated application of 12- O-tetradecanoylphorbol 13-acetate (TPA) and the acute mouse paw oedemas induced by carrageenan and phospholipase A (2) (PLA (2)), and determined its activity on 5-lipoxygenase (5-LOX) and elastase release/activity. Compound 1 reduced the oedema formation, cell infiltration, and tissue damage in the inflammation induced by TPA in mouse ears, along with the acute oedema induced by carrageenan in mouse paws and the acute PLA (2)-induced oedema in mouse paws. The flavone inhibited leukotriene B (4) formation in rat neutrophils and elastase release in human neutrophils, but did not reduce the expression of cyclooxygenase-2 (COX-2) in murine RAW 264.7 macrophages.
CONCLUSIONS:
These experimental results suggest that 1 may act through a direct inhibition of 5-LOX, without affecting the expression of COX-2.
Food Chem. 2011 Jul 15;127(2):394-403.
Chemical composition and biological activity of Citrus jambhiri Lush.[Pubmed:
23140678 ]
The fresh peel of Citrus jambhiri was extracted with aqueous methanol and the residue was fractionated using light petroleum, chloroform and ethyl acetate.
METHODS AND RESULTS:
The constituents of the extracts were separated by column chromatography employing solvents of different polarity. The chemical structure of the isolated compounds was then identified by MS and NMR. Column chromatography of the petroleum fraction resulted in the isolation of nobiletin, 5-O-Demethylnobiletin, tangeretin, 5-hydroxy-3,6,7,8,3',4'-hexamethoxyflavone, 3,5,6,7,8,3',4'-heptamethoxyflavone, and a mixture of β-sitosterol and stigmasterol. The chloroform fraction afforded 6-demethoxynobiletin, 5,4'-dihydroxy-6,7,8,3'-tetramethoxyflavone, limonin and nomilin. The flavonoid glycosides naringin, hesperidin and neohesperidin were isolated from the ethyl acetate fraction. The chemical structure of the isolated compounds was established by MS and NMR (APT, COSY, HSQC, HMBC, and NOESY). LC-ESI-MS analysis of the ethyl acetate fraction afforded eight flavonoid glycosides, while the dichloromethane fraction of the defatted seeds contained seven limonoid aglycones. The chloroform fraction exerted the strongest DPPH(∗) free radical scavenging activity in comparison to other fractions.
CONCLUSIONS:
The petroleum fraction showed a significant inhibition of lipoxygenase indicating an anti-inflammatory action (IC(50) 29±1μg/mL). Some of the isolated polymethoxyflavones exhibited strong cytotoxicity against COS7, HeLa and Caco-2 cell lines.