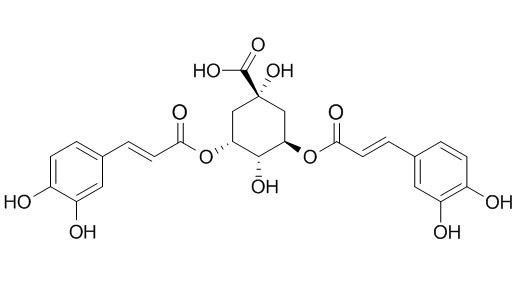
3,5-di-O-caffeoylquinic acid
3,5-di-O-caffeoylquinic acid as a neuraminidase inhibitory ligand in Flos Lonicerae, it has neuroprotective effects on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. 3,5-di-O-caffeoylquinic acid also has antioxidant and anti-complementary activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Plant Sci.2022, 13:982771.
Molecules.2023, 28(8):3490.
Arch Toxicol.2017, 91(10):3225-3245
Front Pharmacol.2021, 12:607403.
Agronomy2023, 13(6), 1435.
Cancer Lett. 2023, 18:216584.
Molecules.2023, 28(13):4907.
Chinese Journal of Tissue Engineering Research2024, 28(8):1149-1154.
Mol Med Rep.2022, 26(4):299.
J.the Korean Socie. Food Sci.&Nut.2023; 52(1):26-39.
Related and Featured Products
Neuroscience. 2010 Sep 1;169(3):1039-45.
Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1.[Pubmed:
20570715 ]
As aged population dramatically increases in these decades, efforts should be made on the intervention for curing age-associated neurologic degenerative diseases such as Alzheimer's disease (AD). Caffeoylquinic acid (CQA), an antioxidant component and its derivatives are natural functional compounds isolated from a variety of plants.
METHODS AND RESULTS:
In this study, we determined the neuroprotective effect of 3,5-di-O-caffeoylquinic acid on Abeta(1-42) treated SH-SY5Y cells using MTT assay. To investigate the possible neuroprotective mechanism of 3,5-di-O-CQA, we performed proteomics analysis, real-time PCR analysis and measurement of the intracellular ATP level. In addition, we carried out the measurement of escape latency time to find the hidden platform in Morris water maze (MWM), real-time PCR using senescence-accelerated-prone mice (SAMP) 8 and senescence-accelerated-resistant mice (SAMR) 1 mice. Results showed that 3,5-di-O-CQA had neuroprotective effect on Abeta (1-42) treated cells. The mRNA expression of glycolytic enzyme (phosphoglycerate kinase-1; PGK1) and intracellular ATP level were increased in 3,5-di-O-CQA treated SH-SY5Y cells. We also found that 3,5-di-O-CQA administration induced the improvement of spatial learning and memory on SAMP8 mice, and the overexpression of PGK1 mRNA.
CONCLUSIONS:
These findings suggest that 3,5-di-O-CQA has a neuroprotective effect on neuron through the upregulation of PGK1 expression and ATP production activation.
Nat Prod Res. 2018 Nov 16:1-8.
Two new phenolic compounds and some biological activities of Scorzonera pygmaea Sibth. & Sm. subaerial parts.[Pubmed:
30445831 ]
METHODS AND RESULTS:
Phytochemical composition of ethyl acetate fraction and total phenolic content, in vitro antioxidant, anti-inflammatory, antimicrobial activities of petroleum ether, chloroform, ethyl acetate and n-butanol fractions of the ethanol extract obtained from the subaerial parts of Scorzonera pygmaea Sibth. & Sm. (Asteraceae) were investigated. Nine compounds; scorzopygmaecoside (1), scorzonerol (2), cudrabibenzyl A (3), thunberginol C (4), scorzocreticoside I (5) and II (6), chlorogenic acid (7), chlorogenic acid methyl ester (8), 3,5-di-O-caffeoylquinic acid (9) were isolated and identified using spectroscopic methods. All substances were isolated for the first time from this species. Compounds 1 and 2 are new.
CONCLUSIONS:
The fractions showed high antioxidant capacity correlated with their phenolic content and no significant antimicrobial activity against tested bacteria and fungi. COX inhibition test was used to evaluate the anti-inflammatory activity and all the fractions showed low inhibition in comparison with indomethacin.
Zhongguo Zhong Yao Za Zhi. 2015 Jan;40(2):269-74.
Anti-complementary phenolic acids from Lonicera japonica.[Pubmed:
26080557]
To study the anti-complementary phenolic acids from Lonicera japonica.
METHODS AND RESULTS:
The anti-complementary activity-directed isolation was carried out with the hemolysis test as guide. All isolation was evaluated for their in vitro anti-complementary activities. The structures were identified by various spectroscopic data including ESI-MS, 1H-NMR, 13C-NMR data.
Fourteen compounds were isolated from the EtOAc fraction of L. japonica extracts, including 8 phenolic acids: 5-O-caffeoylquinic acid (1), chlorogenic (2), 4-O-caffeoylquinic acid (3), 3,5-di-O-caffeoylquinic acid (4), 4,5-di-O-caffeoylquinic acid (5), 3,4-di-O-caffeoylquinic acid (6), caffeic acid (7) and methyl caffeate acid (8); 3 iridoids: secologanoside (9), sweroside (10) and secoxyloganin (11); and 3 flavonoids: luteolin (12), quercetin (13) and kaempferol (14). Compounds 1-9 and 11-14 showed anti-complementary activity in different extents and 3,5-di-O-caffeoylquinic acid (4) exhibited the most significant activity against the classical pathway.
CONCLUSIONS:
Compound 14 is obtained from this plant for the first time, phenolic acids are the main anti-complementary constituents of L. japonica and 3,5-di-O-caffeoylquinic acid(4) is a potential complement inhibitor with strong activity, which worthy to be studied further in the future.
J Chromatogr A. 2018 Sep 21;1568:123-130.
Magnetic beads-based neuraminidase enzyme microreactor as a drug discovery tool for screening inhibitors from compound libraries and fishing ligands from natural products.[Pubmed:
30005943 ]
Neuraminidase (NA) is a glycoside hydrolase that has been proposed as a potential therapeutic target for influenza. Thus, the identification of compounds that modulate NA activity could be of great therapeutic importance. The aim of this study is to develop a drug discovery tool for the identification of novel modulators of NA from both compound libraries and natural plant extracts.
METHODS AND RESULTS:
NA was immobilized onto the surface of magnetic beads and the inherent catalytic activity of NA-functionalized magnetic beads was characterized. Based on the enzymatic activity (hydrolysis ratio), the inhibitory activities of 12 compounds from plant secondary metabolites were screened, and the desired anti-NA activities of flavonoids were certified. Ligand fishing with the immobilized enzyme was optimized using an artificial test mixture consisting of oseltamivir, lycorine and matrine prior to carrying out the proof-of-concept experiment with the crude extract of Flos Lonicerae. The combination of ligand fishing and HPLC-MS/MS identified luteolin-7-O-β-D-glucoside, luteolin, 3,5-di-O-caffeoylquinic acid and 3,4-di-O-caffeoylquinic acid as neuraminidase inhibitory ligands in Flos Lonicerae.
CONCLUSIONS:
This is the first report on the use of neuraminidase functionalized magnetic beads for the identification of active ligands from a botanical matrix, and it sets the basis for the de novo identification of NA modulators from complex biological mixtures.