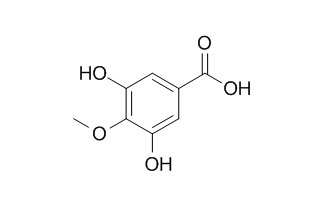
3,5-Dihydroxy-4-methoxybenzoic acid
3,5-dihydroxy-4-methoxybenzoic acid is a COMT inhibitor.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2020, 12(3):595.
Sci Rep.2023, 13(1):13072.
Preprints2017, 2017120176
LWT2020, 130:109535
Chin Med.2022, 17(1):66.
South African J of Plant&Soil2018, 29-32
Phytother Res.2019, 33(7):1784-1793
The Malaysian journal of pathology2019, 41(3):243-251
Int J Mol Sci.2020, 21(9):3144.
Food and Fermentation Industries2019, 45(7):45-51
Related and Featured Products
Rec. Nat. Prod. 2019,13(1): 71-80.
Chemical Constituents and Anti-influenza Viral Activity of the Leaves of Vietnamese Plant Elaeocarpus tonkinensis.[Reference:
WebLink]
Various Elaeocarpus species including Elaeocarpus tonkinensis have been important medicinal plants
that used in traditional medication system and mainly used to cure nervous system-related disorders. However,
their antiviral potential has not been reported yet.
METHODS AND RESULTS:
During the screening of medicinal plant extracts with the
antiviral activity against influenza viruses, we found that E. tonkinensis extract has strong antiviral activity.
Through organic solvent partition and repeated column chromatography using SiO2, C-18 and Sephadex LH-20,
a total of nine compounds were purified from the methanol extract of E. tonkinensis. Their chemical structures
were determined by NMR and MS spectral data to be trolliamide (1), gallic acid (2), urolithin M-5 (3),
hydroquinone (4), 2,4-dihydroxybenzoic acid (5), 3,5-Dihydroxy-4-methoxybenzoic acid (6), corilagin (7),
chebulagic acid (8), and shikimic acid (9). Their antiviral activity against influenza virus strains A/Puerto
Rico/8/34 (H1N1; PR8), A/Hong Kong/8/68 (H3N2; HK) and B/Lee/40 (Lee) was examined on the basis of
cytopathic effect (CPE) assay. Among them, compounds 2, 3, 4, 7, and 8 significantly inhibited viral replication
in a dose-dependent manner with EC50 values ranging from 7.8 to 59.6 μg/mL against influenza A and/or B
viruses with selectivity indices above 5.0.
CONCLUSIONS:
This study suggests that the botanical materials of E. tonkinensis could be promising inhibitors of influenza A and B viruses and applied to the development of a novel herbal medicine.
Toxicology, 1979, 14(3):217-227.
Toxic interaction between narcotic analgesics and inhibitors of catechol-O-methyltransferase.[Reference:
WebLink]
A lethal synergism between morphine and tropolone, an inhibitor of catechol-O-methyltransferase, was previously noted in adult male Holtzman rats.
METHODS AND RESULTS:
The present research demonstrates that this phenomenon generalizes across factors of sex, age, strain (Sprague-Dawley, Wistar) and species (Swiss albino mice). Acute toxicity was also significantly increased (1.5–1.9 times) in the case of codeine, methadone, meperidine and levorphanol, but to a lesser extent than for morphine (4.0 times) in the S-D strain. Another COMT inhibitor, 3,5-Dihydroxy-4-methoxybenzoic acid, interacted with morphine in S-D rats to an equal degree as did tropolone. Post-treatment with 1 mg/kg of naloxone in rats or naltrexone in mice reduced the high lethality associated with morphine plus tropolone. There was a pronounced lowering of whole brain norepinephrine (NE) level after morphine plus tropolone in Wistar rats with doses of each component that alone caused no change in NE. Brain dopamine (DA) was elevated by tropolone and by its combination with morphine. Each drug alone caused slight lowering of brain serotonin. Enhancement by tropolone of the toxicity of (+)-amphetamine in mice and rats was of similar magnitude as for morphine.
CONCLUSIONS:
The possible role of brain NE and/or DA in the sensitivity to acute toxic effects of opioids in rodents is suggested by these data, as well as a parallel in this regard with amphetamine-type stimulants.