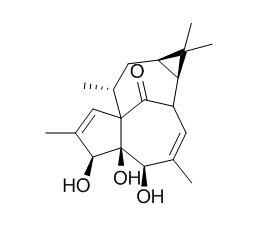
20-Deoxyingenol
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Plant Sci.2023, 14:1207940.
Industrial Crops and Products2020, 146:112186
Phytomedicine.2020, 153440.
Evid Based Complement Alternat Med.2020, 2020:8582318.
Plant J.2021, 107(6):1711-1723.
Journal of Ginseng Research2022, j.jgr.2022.09.005.
Exp Ther Med.2019, 18(6):4388-4396
Turkish Journal of Pharmaceutical Sciences2022, DOI: 10.4274
J Nat Med.2022, 76(1):59-67.
Phytomedicine.2018, 40:37-47
Related and Featured Products
Chem Pharm Bull (Tokyo). 2003 Aug;51(8):935-41.
Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus (part 2).[Pubmed:
12913231]
METHODS AND RESULTS:
Four new ingenane-type diterpenes, 3-O-(2,3-dimethylbutanoyl)-13-O-dodecanoyl-20-O-acetylingenol (1), 3-O-(2,3-dimethylbutanoyl)-13-O-dodecanoyl-20-Deoxyingenol (2), 3-O-(2E,4Z-decadienoyl)-20-Deoxyingenol (3), and 3-O-(2E,4E-decadienoyl)-20-Deoxyingenol (4), two new jatrophane-type diterpenes, kansuinins D (9) and E (10), and four known ingenane-type diterpenes were isolated from the root of Euphorbia kansui. Their structures were elucidated by spectroscopic and chemical analysis, and individual Xenopus cells at the blastular stage were cultured with the diterpenes to test for biological activity.
CONCLUSIONS:
20-Deoxyingenol diterpenes 3 and 4 induced the greatest cell cleavage arrest (0.5 micro g/ml of each compound resulted in >75% cleavage arrest), but cell cleavage inhibitory activity became weak when C-16 had an acyl residue. In contrast, the jatrophane diterpene kansuinin D (9) showed no activity.
Chinese Journal of Natural Medicines, 2016 , 14 (12) :939-945.
Regio- and stereo-selective hydroxylations of ingenane diterpenoids by Mortierella ramanniana and Gibberella fujikuroi.[Reference:
WebLink]
The regio- and stereo-selective hydroxylations of two ingenane diterpenoids, 20-Deoxyingenol (1) and 13-oxyingenol dodecanoat (2), by the filamentous fungi Mortierella ramanniana and Gibberella fujikuroi were investigated in the present study.
METHODS AND RESULTS:
Four undescribed metabolites (3-6) of substrate 1 and two undescribed metabolites (7 and 8) of substrate 2 were isolated. All the metabolites were identified as hydroxylated ingenane derivatives by extensive NMR and HR-ESI-MS data analyses. All the biotransformed compounds and the substrates were evaluated for their cytotoxicities against three human cancer cell lines, including human colon cancer Caco-2, breast cancer MCF-7, and adriamycin (ADM)-resistant MCF-7/ADM cell lines. All ingenane alcohols (1, and 3-6) displayed no significant cytotoxic activities. The substrate 13-oxyingenol dodecanoat (2) showed moderate cytotoxicity with IC50 values being 35.59 ± 5.37 μmol·L-1 (Caco-2), 24.04 ± 4.70 μmol·L-1 (MCF-7), and 22.24 ± 5.19 μmol·L-1 (MCF-7/ADM). However, metabolites 7 and 8 displayed no significant cytotoxicity.
CONCLUSIONS:
These results indicated that the hydroxylation at the C-13 aliphatic acid ester of substrate 2 can significantly reduce the cytotoxic activity.