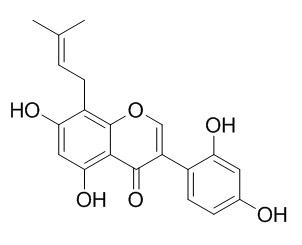
2,3-Dehydrokievitone
2,3- Dehydrokievitone exhibits strong cytotoxic activity and induces apoptosis efficiently in cancer cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2021, 12:652860.
Life Sci.2023, 332:122107.
Nanotechnology.2024, ad470d.
J of Liquid Chromatography & Related Technologies2024, 47(1-5):14-25.
Integr Med Res.2017, 6(4):395-403
Molecules.2018, 23(12):E3103
J Ethnopharmacol.2018, 210:88-94
Appl. Sci. 2021, 11(1),14.
Sci Rep.2024, 14(1):23786.
Kangwon National University2022, 37(1):29-37
Related and Featured Products
Rec. Nat. Prod., 2016, 10(4):441-51.
Antioxidative and Antitumor Effects of Isoflavones Isolated from the Leaves of Maackia fauriei [Reference:
WebLink]
The flowers of Maackia fauriei have traditionally been used to treat hypertension, apoplexy, hemostasis, vaginal bleeding, and dystocia; moreover, the bark of this plant has been used as a natural dye. In the present study, activity-guided isolation of the leaves of M. fauriei yielded five isoflavones [genistein (1), pratensein (2), genistin (3), 2'-hydroxygenistein-7-O-β-D-glucopyranoside (4), and 2,3-Dehydrokievitone (5)]; three pterocarpans [medicarpin (6), maackiain (7), and 4-hydroxy maackiain (8)]; and one flavonol [isoquercitrin (9)].
METHODS AND RESULTS:
To evaluate the anti-oxidative effects of these compounds, their 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assays and nitrotetrazolium blue chloride (NBT) superoxide scavenging assays were measured. And the anti-tumor activity against human cancer cell lines in genital system, LNCaP, PC-3,HeLa and OVCAR-3 cells were evaluated by MTT method. Furthermore, the apoptosis of the PC-3 and HeLa cells were determined by by annexin V-FITC and PI their fluorescence was analyzed by flow cytometry. The flavonol (9, isoquercitrin) and pterocarpan (8, 4-hydroxymaackiain) showed strong anti-oxidative activities. Besides, the isoflavones (1-5) did not showed anti-oxidative activity and the isoflavones (1-5) and pterocarpans (6-8) generally showed the potent cytotoxic activity against all of four human genital cancer cells. Especially, 2,3-Dehydrokievitone (5) which had a prenyl group at C-8 position of the A-ring exhibited strong cytotoxic activity and induced apoptosis efficiently in cancer cells.
Phytochemistry. 2000 Nov;55(5):457-9.
Two isoflavanones from the stem bark of Erythrina sacleuxii.[Pubmed:
11140607]
METHODS AND RESULTS:
From the stem bark of Erythrina sacleuxii two new isoflavanones, (R)-5,7-dihydroxy-2',4',5'-trimethoxyisoflavanone (trivial name, (R)-2,3-dihydro-7-demethylrobustigenin) and (R)-5-hydroxy-2',4',5'-trimethoxy-2",2"-dimethylpyrano[5",6":6,7]isoflavanone (trivial name, (R)-saclenone) were isolated. In addition the known compounds shinpterocarpin, 2,3-Dehydrokievitone, abyssinone V, abyssinone V-4'-methyl ether, erythrinasinate and 4'-O-methylsigmoidin B were isolated.
CONCLUSIONS:
The structures were determined on the basis of spectroscopic evidence.