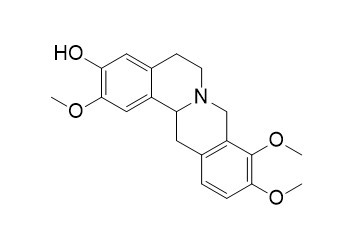
Tetrahydrojatrorrhizine
Corypalmine, an alkaloid isolated from Stephania cepharantha, is an inhibitor of prolyl endopeptidase/oligopeptidase (PREP/POP) with IC50 of 128.0 μM. Corypalmine is an antifungal.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Immunol. 2020, 11:62.
Natural Product Communications2020, doi: 10.1177.
Industrial Crops and Products2018, 353-362
Antioxidants (Basel).2020, 9(11):1121.
Animals (Basel).2024, 14(20):2990.
Molecules.2018, 23(2)
Enzyme Microb Technol.2019, 122:64-73
Plant Cell, Tissue and Organ Culture (PCTOC)2024, 158:54
Phytomedicine Plus2024, 4(4): 100655.
Int J Mol Sci.2017, 18(5)
Related and Featured Products
Folia Microbiol (Praha) . 2002;47(3):287-290.
Efficacy of alkaloid (-)-corypalmine against spore germination of some fungi[Pubmed:
12094740]
Inhibition activity of the alkaloid (-)-corypalmine on spore germination of plant pathogenic and saprophytic fungi (Alternaria solani, A brassicicola, A. brassicae, A. melongenae, Curvularia pallescens, C. lunata, C. maculans, Curvularisa sp., Colletotrichum sp., Helminthosporium speciferum, H. frumentacei, H. pennisetti, Heterosporium sp., Penicillum sp., Ustilago cynodontis) was determined. Spore germination of all the tested fungi was inhibited, Heterosporium sp. and Ustilago cynodontis being the most sensitive (complete inhibition of spore germination was observed at the very low concentration of 200 ppm). Curvularia palliscens, C. maculans and Curvularia sp. were less sensitive; complete inhibition of spore germination occurred at 400 ppm.
An Acad Bras Cienc . 2017;89(3 Suppl):2053-2073.
Cytotoxicity and bacterial membrane destabilization induced by Annona squamosa L. extracts[Pubmed:
28813096]
This study aimed to further investigate the cytotoxicity against tumor cell lines and several bacterial strains of Annona squamosa and its mode of action. Methanol extracts of A. squamosa leaves (ASL) and seeds (ASS) were used. ASL showed significant antibacterial activity against S. aureus, K. pneumoniae and E. faecalis with MIC values of 78, 78 and 39 μg/mL respectively. Moreover, ASL exhibited significant biofilm disruption, rapid time dependent kinetics of bacterial killing, increased membrane permeability and significantly reduced the cell numbers and viability. Regarding the cytotoxicity against tumor cell lines, ASS was more active against Jurkat and MCF-7 cells, with CI50 1.1 and 2.1 μg/mL, respectively. ASL showed promising activity against Jurkat and HL60, with CI50 4.2 and 6.4 μg/mL, respectively. Both extracts showed lower activity against VERO cells and reduced the clonogenic survival at higher concentrations (IC90) to MCF-7 and HCT-116 lineages. The alkaloids anonaine, asimilobine, corypalmine, liriodenine nornuciferine and reticuline were identified in extracts by UPLC-ESI-MS/MS analysis. This study reinforced that A. squamosa presents a remarkable phytomedicinal potential and revealed that its antimicrobial mechanism of action is related to bacterial membrane destabilization.
J Nat Prod . 2009 Aug;72(8):1516-1519.
7,7-Dimethylaporphine alkaloids from the stem of Guatteriopsis friesiana[Pubmed:
19639965]
Phytochemical investigation of a methanolic extract of the stem of Guatteriopsis friesiana afforded two new 7,7-dimethylaporphine alkaloids, 6,6a-dihydrodemethoxyguadiscine (1) and guatteriopsiscine (3), together with demethoxyguadiscine (2), liriodenine (4), corypalmine (5), and coreximine (6). Their structures were elucidated on the basis of spectroscopic methods (UV, IR, EIMS, HRESIMS, 1D/2D NMR). The absolute configurations of 1 and 3 were determined from the circular dichroism curves. The presence of 7,7-dimethylaporphine alkaloids in this species is important for the chemotaxonomy of Guatteriopsis. Antimicrobial activity of compounds 1-5 was investigated, and 4 showed activity against Rhodococcus equi, with a MIC value of 10 microg x mL(-1).
Zhongguo Zhong Yao Za Zhi . 2010 May;35(10):1272-1275.
[Alkaloids in stems and leaves of Stephania cepharantha][Pubmed:
20707195]
Objective: To study the alkaloids in the stems and leaves of Stephania cepharantha.
Method: The dried stems and leaves of S. cepharantha were percolated with 95% ethanol and the solvent was removed by rotary evaporation to give a concentrate, and the concentrate was extracted by petroleum ether and chloroform. Column chromatograghy on MCI CHP 20P, silica gel, Rp-18, Sephadex LH-20 and polyamide were applied for the isolation and purification of the chloroform fraction. The structures were elucidated by their physicochemical properties and spectral data.
Result: Eleven alkaloids were obtained and identified as lysicamine (1), tetrahadropalmatine (2), palmatine (3), isocorydione (4), corydalmine (5), corypalmine (6), sinoracutine (7), sinoacutine (8), cepharamine (9), isocorydine (10) and corydine (11).
Conclusion: Compounds 2-7 were isolated from S. cepharantha for the first time, and compound 7 was isolated from the genus Stephania for the first time, compound 4 was isolated from the Menispermaceae family for the first time.
J Nat Prod . 2009 Aug;72(8):1516-1519.
7,7-Dimethylaporphine alkaloids from the stem of Guatteriopsis friesiana[Pubmed:
19639965]
Phytochemical investigation of a methanolic extract of the stem of Guatteriopsis friesiana afforded two new 7,7-dimethylaporphine alkaloids, 6,6a-dihydrodemethoxyguadiscine (1) and guatteriopsiscine (3), together with demethoxyguadiscine (2), liriodenine (4), corypalmine (5), and coreximine (6). Their structures were elucidated on the basis of spectroscopic methods (UV, IR, EIMS, HRESIMS, 1D/2D NMR). The absolute configurations of 1 and 3 were determined from the circular dichroism curves. The presence of 7,7-dimethylaporphine alkaloids in this species is important for the chemotaxonomy of Guatteriopsis. Antimicrobial activity of compounds 1-5 was investigated, and 4 showed activity against Rhodococcus equi, with a MIC value of 10 microg x mL(-1).