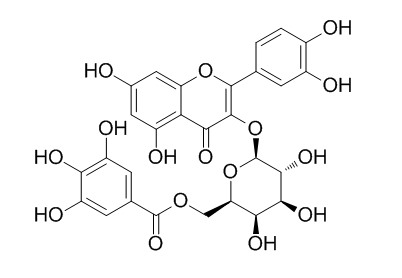
Quercetin 3-O-(6''-galloyl)-beta-D-galactopyranoside
Quercetin 3-O-(6''-galloyl)-beta-D-galactopyranoside can effectively induce apoptosis via p53, MAPKs and the mitochondrial apoptotic pathways.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2019, 236:31-41
Biomed Chromatogr.2016, 30(10):1573-81
Vietnam Journal of Food Control2022, 5(3):pp.390-401.
Journal of Ginseng Research2022, j.jgr.2022.09.005.
Int J Mol Sci.2019, 20(3):E651
Natural Product Communications2021, 16(9):1-10.
Biomedicines.2024, 12(12):2928.
Pharmaceuticals (Basel).2021, 14(8):742.
Nat Prod Sci.2018, 24(3):206
Virulence.2018, 9(1):588-603
Related and Featured Products
Food Funct. 2015 Dec;6(12):3746-59.
Bioactive compounds of Eriocaulon sieboldianum blocking proliferation and inducing apoptosis of HepG2 cells might be involved in Aurora kinase inhibition.[Pubmed:
26369427 ]
Eriocaulon sieboldianum (Sieb. & Zucc. ex Steud.) is an edible and medicinal plant used in traditional Chinese medicine. Often in combination with other herbs, it is processed into healthcare beverages for expelling wind-heat, protecting eyes, and reducing blood lipids. Besides, its water decoction together with other herbs has been utilized to treat cancer in China. However, the active ingredients and the precise cellular mechanisms of E. sieboldianum remain to be elucidated.
The Aurora kinase family plays critical roles in the regulation of cell division and has attracted great attention to the identification of small-molecule Aurora kinase inhibitors for potential treatment of cancer.
METHODS AND RESULTS:
A molecular docking study was employed for docking of the most bioactive compounds. Hispidulin (HPDL) and quercetin-3-O-(6''-O-galloyl)-β-D-galactopyranoside (Quercetin 3-O-(6''-galloyl)-beta-D-galactopyranoside, QGGP) were singled out as potent inhibitors of Aurora kinase. Their inhibitory activity towards Aurora kinase was further confirmed by the obvious decrease in autophosphorylation of Aurora-A (Thr288) and Aurora-B (Thr232). Moreover, the induction of cell cycle arrest in HepG2 cells and the suppressed phosphorylation of histone H3 were also consistent with the inhibition of Aurora kinase. The data indicate that the E. sieboldianum extract and its two active compounds, HPDL and QGGP, could effectively induce apoptosis via p53, MAPKs and the mitochondrial apoptotic pathways.
CONCLUSIONS:
These findings could improve the understanding and enhance the development of drugs based on E. sieboldianum and raise its application value in anticancer therapy or prevention. In addition, our results indicated that Aurora kinase might be a novel target of HPDL and QGGP.
Yao Xue Xue Bao. 2010 Mar;45(3):334-7.
One new galloyl glycoside from fresh leaves of Psidium guajava L.[Pubmed:
21348423]
To investigate the chemical constituents of Psidium Guajava L, the EtOH/H2O extract of the fresh leaves was subjected to various chromatography.
METHODS AND RESULTS:
Five constituents with galloyl moiety were isolated and elucidated as 1-O-(1, 2-propanediol)-6-O-galloyl-beta-D-glucopyranoside (1), gallic acid (2), ellagic acid (3), ellagic acid-4-O-beta-D-glucopyranoside (4) and Quercetin 3-O-(6''-galloyl)-beta-D-galactopyranoside (5) by spectroscopic methods, including 2D NMR and HR-ESI-MS spectrometry as well as by comparison with published data.
CONCLUSIONS:
Compounds 4 and 5 were obtained from P. guajava for the first time, and compound 1 is a new polyhydroxyl compound.