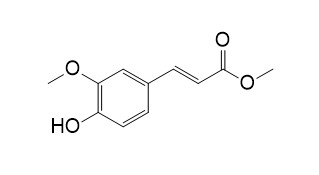
Methyl 4-hydroxy-3-methoxycinnamate
Methyl 4-hydroxy-3-methoxycinnamate is an inhibitor from wheat rust uredospores.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int Immunopharmacol.2021, 101(Pt A):108181.
Front Pharmacol.2019, 10:1355
Biomedicines.2021, 9(8):954.
Food Analytical Methods2020, 1-10
J Ethnopharmacol.2016, 192:370-381
J of the Korean Society of Food Science and Nutrition2016, 45(7):1017-1025
Drug Chem Toxicol.2020, 1-12.
Phytomedicine.2018, 47:48-57
Journal of Apiculture2023.38(3):249-254.
Chem Biodivers.2023, 20(10):e202300741.
Related and Featured Products
Science, 1971, 173(3999):835-836.
Identification of the Germination Self-Inhibitor from Wheat Stem Rust Uredospores.[Pubmed:
17812195]
METHODS AND RESULTS:
Two germination inhibitors from wheat rust uredospores were identified as the cis and trans isomers of Methyl 4-hydroxy-3-methoxycinnamate (methyl ferulate). They are the self-inhibitors from these spores described previously.
Febs Journal, 2010, 248(1):245-251.
Methyl phenylalkanoates as substrates to probe the active sites of esterases.[Pubmed:
9310385]
METHODS AND RESULTS:
We have used methyl esters of phenylalkanoic acids to probe the active site of two esterases (FAE-III and CinnAE) from Aspergillus niger. Only
Methyl 4-hydroxy-3-methoxycinnamate and 4-hydroxy-3-methoxyphenylpropionate out of 19 substrates tested were significant substrates for both enzymes (k(cat) values about 10(2) s(-1) and 10(3) s(-1), respectively). Lengthening or shortening the aliphatic side chain while maintaining the same aromatic substitutions completely abolished activity for both enzymes, which demonstrates the importance of the correct distance between the aromatic group and the ester bond. Differences in Km values for FAE-III were small (0.45-2.08 mM) but there were two orders of magnitude difference in k(cat) values (12.1-1063 s(-1)), whereas for CinnAE, there were large differences in values for both Km (0.014-1.32 mM) and k(cat) (41.3-1410 s(-1)). Lability of the ester bonds, as estimated from second-order rate constants (k2) for chemical reaction with sodium hydroxide, did not correlate to k(cat) for CinnAE (r = 0.33) or for FAE-III (r = 0.43). Maintaining the phenylpropenoate structure but altering the substitutions on the aromatic ring demonstrated the following: a 3-methoxy group is essential for FAE-III activity, whereas a 3-methoxy group precluded activity of CinnAE, with the exception of Methyl 4-hydroxy-3-methoxycinnamate which was a relatively poor substrate for CinnAE; (b) increasing the number of methoxy substitutions increased the activity of FAE-III, and decreased the activity of CinnAE; (c) 4-hydroxy substituents, and additional hydroxy substituents, increased the activity of CinnAE, but decreased that of FAE-III; (d) the rate of hydrolysis with sodium hydroxide of the methyl esters in general is decreased by hydroxy substitutions on the aromatic ring but increased by methoxy substitutions. Analysis of kinetic data obtained in the presence of inhibitors indicated that substrate analogs were able to bind to both free CinnAE and to a CinnAE-substrate complex, but conversely, were only able to bind to free FAE-III.
CONCLUSIONS:
The results show that the specificities of the two A. niger esterases are complementary. The rate of hydrolysis by this class of carboxylic ester hydrolase does not depend on the intrinsic lability of the ester bond, but depends on both the distance between the aromatic ring and the ester bond, and the substitutions on the aromatic ring.
Phytotherapy Research, 2006,20(10):840-843.
Inhibition of respiratory burst in human neutrophils and lipoxygenase enzyme by compounds from Haloxylon griffithii.[Reference:
WebLink]
METHODS AND RESULTS:
Secondary metabolites, ferulic acid (1), 2,6‐dimethoxy‐4‐hydroxy acetophenone (2), herniarin (3), p‐hydroxy acetophenone (4),
methyl 3,4‐dihydroxycinnamate (5), and
Methyl 4-hydroxy-3-methoxycinnamate (6) were isolated from Haloxylon griffithii, a member of the family Chenopodiaceae. The structures of compounds 1–6 were identified with the help of spectroscopic techniques. These compounds were isolated for the first time from this plant. The lipoxygenase and respiratory burst inhibitory activities were determined.
CONCLUSIONS:
Compound 5 was found to be the most potent inhibitory activity against respiratory burst in human neutrophils among all the compounds as well as exhibited moderate lipoxygenase inhibitory activity from this plant.