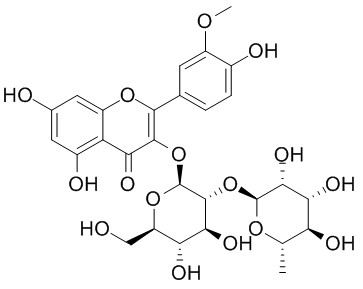
Isorhamnetin-3-O-neohespeidoside
Isorhamnetin 3-O-neohesperoside is the major active substance of Puhuang, a traditional herb medicine widely used in clinical practice to tackle many chronic diseases. It has significant biological and pharmacological activities, including antioxidant, antiatherogenic and antimicrobial effects.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
RSC Adv.2018, 32621-32636
Exp Biol Med (Maywood).2019, 244(18):1665-1679
J Pharmaceut Biomed2020, 182:113110
Cell Death Differ.2021, 1-8.
Plant Physiol Biochem.2023, 202:107913.
Nutrients.2023, 15(12):2644.
Molecules.2023, 28(10):4062.
J of Apicultural Research2020, 10.1080
J Korean Med Obes Res.2023, 23:10-7
Jurnal Ilmu Pertanian Indonesia2023, 28(4):525-533.
Related and Featured Products
J Sep Sci . 2019 Jul;42(14):2426-2434.
A rapid and efficient extraction method based on industrial MCM-41-miniaturized matrix solid-phase dispersion extraction with response surface methodology for simultaneous quantification of six flavonoids in Pollen typhae by ultra-high-performance liquid chromatography[Pubmed:
31077572]
Abstract
An industrial MCM-41-miniaturized matrix solid-phase dispersion extraction coupled with response surface methodology was explored to determine L-epicatechin, typhaneoside, Isorhamnetin-3-O-neohespeidoside, naringenin, kaempferol, and isorhamnetin in Pollen typhae by ultra-high performance liquid chromatography connected to a photodiode array detection. Several variables were optimized in detail, including mesh number of sieve, type of adsorbent, mass ratio of sample to adsorbent, grinding time, methanol concentration, and elution volume. Central composite design was applied to optimize the best conditions for the maximum yields of the total flavonoids. The results displayed a good linear relationship (R > 0.9992) and the recoveries ranged from 92.9 to 103% (RSD < 4.53%) of the six flavonoids. The optimal method with high efficiency and low consumption was obviously better than heating reflux and ultrasonic extraction. It was proven that the developed industrial MCM-41-miniaturized matrix solid-phase dispersion extraction coupled with simple ultra-high performance liquid chromatography method could be a rapid and efficient tool for extraction and determination of flavonoids in natural products.
Keywords: MCM-41; miniaturized matrix solid-phase dispersion extraction; response surface methodology; traditional Chinese medicine; ultra-high-performance liquid chromatography.
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Jan 1;974:131-7.
Measurement of hydroxysafflor yellow A in human urine by liquid chromatography-tandem mass spectrometry.[Pubmed:
25463208]
METHODS AND RESULTS:
A rapid and specific high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed for the quantification of hydroxysafflor yellow A (HSYA) in human urine with Isorhamnetin-3-O-neohespeidoside as internal standard (IS). HSYA and IS were extracted from urine samples by simple solid-phase extraction and separated on an Agilent Zorbax SB C18 column (4.6 mm × 150 mm, 5 μm) with the mobile phase of 0.2 mM ammonium acetate: methanol (30/70, v/v) at a flow rate of 0.4 mL/min. Polar endogenous interferences eluted in 0.1-2.5 min were switched into waste channel by the Valve Valco, to reduce the possible matrix effect for MS detection in each run. The MS detection of analytes was performed on a tandem mass spectrometer equipped with an electrospray ionization source in negative mode using multiple-reaction monitoring. The MS/MS ion transitions monitored were m/z 611.3→491.2 for HSYA and m/z 623.2→299.2 for IS. The method was fully validated for selectivity, sensitivity, linearity, precision, accuracy, recovery, matrix effect and stability, and then was applied to the urinary excretion study of injectable powder of pure HSYA in healthy Chinese volunteers for the first time.
CONCLUSIONS:
The results suggested that urine was the main excretion way of HSYA in healthy volunteers, further demonstrating the feasibility and necessity of our current method.