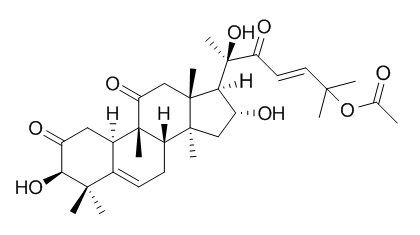
Isocucurbitacin B has significantly activities against HeLa and HT-29 human cancer cells with IC50 values ranging from 0.93 to 9.73uM.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol. 2015 Jul 1;169:18-23.
Bioassay-guided isolation and identification of cytotoxic compounds from Bolbostemma paniculatum.[Pubmed:
25882313 ]
Four cucurbitacine triterpenoid sapogenins, Isocucurbitacin B(1), 23,24-dihydroIsocucurbitacin B(2), cucurbitacin E(3), 23,24-dihydrocucurbitacin E(4), and 11 triterpenoid saponins, tubeimosideI(5), tubeimoside III(6), tubeimoside V(7), dexylosyltubeimoside III(8), lobatoside C(9), tubeimoside A(10), tumeimoside B(11), lobatoside A(12), tubeimoside C(13), tubeimoside IV(14), 7β,18,20,26-tetrahydroxy-(20S)-dammar-24E-en-3-O-α-L-(4-acetyl)arabinopyranosyl-(1→2)-β-D-glucopyranoside(15) were isolated from the active EtOAc and n-BuOH extracts.
METHODS AND RESULTS:
Of them, compounds 2, 4, 9 and 12 were firstly isolated from the Bolbostemma genus. MTT assay revealed that compounds 1, 3 and 4 had significantly activities against HeLa and HT-29 human cancer cells with IC50 values ranging from 0.93 to 9.73μM. It is worth mentioning that compound 4׳s activities against the two cell lines are 12- and 8-fold that of the positive control drug (5-Fu). Whereas, the cyclic bisdesmosides 5-9 exerted significantly activities on BGC-823, HeLa, HT-29 and MCF-7 cancer cells with IC50 values ranging from 1.30 to 15.64μM. And 6׳s activities against the four cell lines are 6-, 3-, 10- and 16-fold that of 5-Fu and 8׳s activities against the four cell lines are 5-, 3-, 14- and 9-fold that of 5-Fu.
CONCLUSIONS:
The cytotoxic activity of the bulbs of B. paniculatum is mainly ascribable to cucurbitacine triterpenoid sapogenins (1-4) and the cyclic bisdesmosides (5-9). The cyclic bisdesmosides are the main anti-cancer active compounds of B. paniculatum. The above results provide scientific evidence to support, to some extent, the ethnomedicinal use of B. paniculatum as anticancer remedies in traditional Chinese medicine.