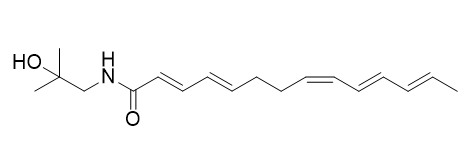
Hydroxy-gamma-sanshool
Hydroxy-γ-sanshool is a pungent compound, it can cause oral numbness.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Med Rep.2024, 29(2):26.
Asian J Beauty Cosmetol2016, 14(3):249-257
Mol Nutr Food Res.2024, 68(20):e2400414.
Anal Bioanal Chem.2020, 412(12):3005-3015.
ScienceAsia2024, 50,2024073:1-9
Plant Foods Hum Nutr.2021, 76(4):472-477.
Sci Adv.2018, 4(10)
Natural Product Communications2020, doi: 10.1177.
Molecules.2015, 20(10):19172-88
Research Square2021, 10.21203.
Related and Featured Products
Journal of the science of food and agriculture, 2019, 99:págs. 1475-1483.
The relationship between alkylamide compound content and pungency intensity of Zanthoxylum bungeanum based on sensory evaluation and ultra‐performance liquid chromatography‐mass spectrometry/ mass spectrometry (UPLC‐MS/MS) analysis.[Reference:
WebLink]
METHODS AND RESULTS:
The pungency intensity of 19 Zanthoxylum bungeanum samples was first determined with Scoville pungency units (SPUs). The SPUs were found to range from 3.80E + 04 to 5.40E + 05. The chemical compositions and contents were measured next, using the ultra‐performance liquid chromatography‐mass spectrometry/ mass spectrometry (UPLC‐MS/MS) method. The total alkylamide content ranged from 9.83 ± 0.15 to 89.98 ± 1.35 g kg−1. Hydroxy‐ϵ‐sanshool, hydroxy‐α‐sanshool, Hydroxy-beta-sanshool, Hydroxy-γ-sanshool(Hydroxy-gamma-sanshool), bungeanool, and isobungeanool were found to be the key pungent compounds, ranging in proportion from 92.65% to 97.69%. The relationship between alkymide compound content and pungency intensity was also analyzed by ridge regression, and it was found that the β values of independent variables were stable when k was more than 0.6. The regression coefficients of hydroxy‐ϵ‐sanshool, hydroxy‐α‐sanshool, hydroxy‐β‐sanshool, hydroxy‐γ‐sanshool, bungeanool, isobungeanool, and other alkylamides were 0.105, 0.177, 0.386, −0.166, −0.006, 0.005, and −0.018, respectively.
CONCLUSIONS:
Hydroxy‐ sanshool compounds were important in determinant the pungency intensity of Z. bungeanum. Knowledge of the relationship between alkymide compound content and pungency intensity will assist in the creation of new methods to determine pungency intensity and provide a scientific basis for flavor design, development of pungent food products, and consumer choice evaluations.
Planta Med. 1990 Feb;56(1):89-91.
Lignans and other constituents from South and central american zanthoxylum species.[Pubmed:
17221375 ]
METHODS AND RESULTS:
Eight lignans (+/-)-syringaresinol, (-)-pinoresinol, (+)-sesamin, (+)-eudesmin, (+)-epieudesmin, (-)-asarinin, (-)-matairesinol, (-)-kobusin, two terpenes lupeol, beta-sitosterol, one aliphatic unsaturated amide, Hydroxy-gamma-sanshool(Hydroxy-γ-sanshool), and one alkaloid, magnoflorine, were isolated from ZANTHOXYLUM species of Central and South America.
METHODS AND RESULTS:
Their structures were elucidated mainly by (1)H-, (13)C-NMR, and mass spectroscopy.